E X T O X N E T
Extension Toxicology Network
Toxicology Information Briefs
A Pesticide Information Project of Cooperative Extension Offices of Cornell University, Oregon State University, the University of Idaho, and the University of California at Davis and the Institute for Environmental Toxicology, Michigan State University. Major support and funding was provided by the USDA/Extension Service/National Agricultural Pesticide Impact Assessment Program.
EXTOXNET primary files maintained and archived at Oregon State University
Revised 9/93.
MOVEMENT OF PESTICIDES IN THE ENVIRONMENT

INTRODUCTION
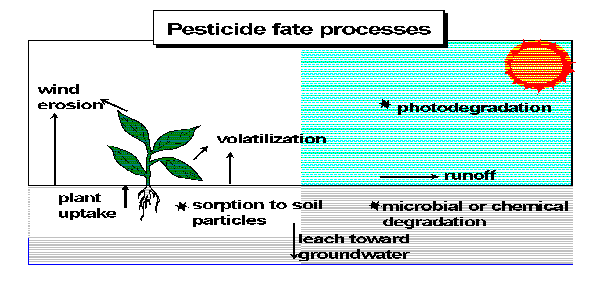
The widespread use and disposal of pesticides by farmers, institutions and the general public provide many possible sources of pesticides in the environment. Following release into the environment, pesticides may have many different fates. Pesticides which are sprayed can move through the air and may eventually end up in other parts of the environment, such as in soil or water. Pesticides which are applied directly to the soil may be washed off the soil into nearby bodies of surface water or may percolate through the soil to lower soil layers and groundwater. Pesticides which are injected into the soil may also be subject to the latter two fates. The application of pesticides directly to bodies of water for weed control, or indirectly as a result of leaching from boat paint, runoff from soil or other routes, may lead not only to build up of pesticides in water, but also may contribute to air levels through evaporation.
This incomplete list of possibilities suggests that the movement of pesticides in the environment is very complex with transfers occurring continually among different environmental compartments. In some cases, these exchanges occur not only between areas that are close together (such as a local pond receiving some of the herbicide application on adjacent land) but also may involve transportation of pesticides over long distances. The worldwide distribution of DDT and the presence of pesticides in bodies of water such as the Great Lakes far from their primary use areas are good examples of the vast potential of such movement.
While all of the above possibilities exist, this does not mean that all pesticides travel long distances or that all compounds are threats to groundwater. In order to understand which ones are of most concern, it is necessary to understand how pesticides move in the environment and what characteristics must be considered in evaluating contamination potential. Two things may happen to pesticides once they are released into the environment. They may be broken down, or degraded, by the action of sunlight, water or other chemicals, or microorganisms, such as bacteria. This degradation process usually leads to the formation of less harmful breakdown products but in some instances can produce more toxic products.
The second possibility is that the pesticide will be very resistant to degradation by any means and thus remain unchanged in the environment for long periods of time. The ones that are most rapidly broken down have the shortest time to move or to have adverse effects on people or other organisms. The ones which last the longest, the so-called persistent pesticides, can move over long distances and can build up in the environment leading to greater potential for adverse effects to occur.
PROPERTIES OF PESTICIDES
In addition to resistance to degradation, there are a number of other properties of pesticides which determine their behavior and fate. One is how volatile they are; that is, how easily they evaporate. The ones that are most volatile have the greatest potential to go into the atmosphere and, if persistent, to move long distances. Another important property is solubility in water; or how easily they dissolve in water. If a pesticide is very soluble in water, it is more easily carried off with rainwater, as runoff or through the soil as a potential groundwater contaminant (leaching). In addition, the water-soluble pesticide is more likely to stay mixed in the surface water where it can have adverse effects on fish and other organisms. If the pesticide is very insoluble in water, it usually tends to stick to soil and also settle to the bottoms of bodies of surface water, making it less available to organisms.
ENVIRONMENTAL CHARACTERISTICS
From a knowledge of these and other characteristics, it is possible to predict in a general sense how a pesticide will behave. Unfortunately, more precise prediction is not possible because the environment itself is very complex. There are, for example, huge numbers of soil types varying in the amount of sand, organic matter, metal content, acidity, etc. All of these soil characteristics influence the behavior of a pesticide so that a pesticide which might be anticipated to contaminate groundwater in one soil may not do so in another.
Similarly, surface waters vary in their properties, such as acidity, depth, temperature, clarity (suspended soil particles or biological organisms), flow rate, and general chemistry. These properties and others can affect pesticide movement and fate. Everyone is familiar with the difficulties of forecasting weather, which is partly due to problems in predicting air flow patterns. As a result, determination of pesticide distribution in the atmosphere is subject to great uncertainty.
With such great complexity, scientists cannot determine exactly what will happen to a particular pesticide once it has entered the environment. However, they can divide pesticides into general categories with regard to, for example, persistence and potential for groundwater contamination and they can also provide some idea as to where the released pesticide will most likely be found at its highest levels. Thus, it is possible to gather information which can help make informed decisions about what pesticides to use in which situations and what possible risks are being faced due to a particular use.
MOVEMENT OF PESTICIDES IN SOIL
The table below lists some of the more commonly used pesticides with an estimate of their persistence in soil. In this table, persistence is measured as the time it takes for half of the initial amount of a pesticide to breakdown. Thus, if a pesticide's half-life is 30 days, half will be left after 30 days, one-quarter after 60 days, one-eighth after 90 days and so on. It might seem that a short half-life would mean a pesticide would not have a chance to move far in the environment. This is generally true; however, if it is also very soluble in water and the conditions are right, it can move rapidly through certain soils. As it moves away from the surface, it moves away from the agents which are degrading it such as sunlight and bacteria. As it gets deeper into the soil, it degrades more slowly and thus has a chance to get into groundwater. Our measures of soil persistence only describe pesticide behavior at or near the surface.
The downward movement of non-persistent pesticides is not an unlikely scenario and several pesticides with short half-lives, such as aldicarb, have been widely found in groundwater. In contrast, very persistent pesticides may have other properties which limit their potential for movement throughout the environment. Many of the chlorinated hydrocarbon pesticides are very persistent and slow to breakdown but also very water insoluble and tend not to move down through the soil into groundwater. They can, however, become problems in other ways since they remain on the surface for a long time where they may be subject to runoff and possible evaporation. Even if they are not very volatile, the tremendously long time that they persist can lead, over time, to measurable concentrations moving through the atmosphere and accumulating in remote areas.
PESTICIDE PERSISTENCE IN SOILS
| Low Persistence (half-life 30 days) | Moderate Persistence (half-life 30-100 days) | High Persistence (half-life >100 days) |
|
|
|
ROLE OF LIVING ORGANISMS
So far, the discussion has focused on air, soil and water. However, living organisms may also play a significant role in pesticide distribution. This is particularly important for pesticides which can accumulate in living creatures. An example of accumulation is the uptake of a very water-insoluble pesticide, such as chlordane, by a creature living in water. Since this pesticide is stored in the organism, the pesticide accumulates and levels increase over time. If this organism is eaten by a higher organism which also can store this pesticide, levels can reach higher values in the higher organism than is present in the water in which it lives. Levels in fish, for example, can be tens to hundreds of thousands of times greater than ambient water levels of the same pesticide. This type of accumulation is called bioaccumulation.
In this regard, it should be remembered that humans are at the top of the food chain and so may be exposed to these high levels when they eat food animals which have bioaccumulated pesticides and other organic chemicals. It is not only fish but also domestic farm animals which can be accumulators of pesticides and so care must be used in the use of pesticides in agricultural situations.
SUMMARY
The release of pesticides into the environment may be followed by a very complex series of events which can transport the pesticide through the air or water, into the ground or even into living organisms. The most important route of distribution and the extent of distribution will be different for each pesticide. It will depend on the formulation of the pesticide (what it is combined with) and how and when it is released. Despite this complexity, it is possible to identify situations that can pose concern and to try to minimize them. However, there are significant gaps in the knowledge of pesticide movement and fate in the environment and so it is best to minimize unnecessary release of pesticides into the environment. The fewer pesticides that are unnecessarily released, the safer our environment will be.
DISCLAIMER: The information in this brief does not in any way replace or supersede the information on the pesticide product label/ing or other regulatory requirements. Please refer to the pesticide product label/ing.