

 Environmental Toxicology
Newsletter
Environmental Toxicology
Newsletter
"Published Occasionally at Irregular Intervals"
~ Dr.
Arthur L. Craigmill ~
Extension
Toxicologist
Vol. 26 No. 2 -- July 2006
 "IN THIS ISSUE"
"IN THIS ISSUE"
Introduction
This newsletter contains the latest news
release from the California Department of Pesticide Regulation (DPR)
concerning the 2004 Pesticide Use Report Summary. When you read
this news release (and the report also if you link to it) please
remember that the number of pounds of pesticide used, and the number of
acres treated are very general and non-specific measures of the overall
impact that a pesticide may have on the environment and human health.
Remember the paraphrase of Paracelsus which Dr. Alice Ottoboni coined
and used as the title of her book, "The Dose Makes the Poison".
Remember also the words of our former Chancellor at UCD, Dr. Emil Mrak
who wrote; "There are no harmless substances, only harmless ways of
using substances."
I would like to challenge our readers and
contributors (involuntary as they are) about the use of several
"buzz-words." These descriptive terms include "reduced risk" and "least
toxic." What do they mean? What measure of risk is reduced?
What aspect of toxicity is being referred to for comparison? To
me these are ill-defined, "feel-good" words that I would expect to hear
in commercials rather than a news release or report from a state
agency. I would also like to challenge all of our readers to use
appropriate terminology in their descriptions of toxicants. The
DPR press release refers to "chemicals classified as reproductive
toxins", and I am certain that this report does not refer to just
toxins, but to human-made chemicals as well. Toxins are toxicants
of biological origin, and the term implies that a toxin comes from a
living source. BT toxin is a toxin. Carbaryl is not a toxin, and most
chemical pesticides are not toxins. Our mission is to educate and
inform people, so please, let's start being precise and use scientific
terminology appropriately. Thank you.
I would also like to announce that beginning July 1,
I will be taking over a 40% appointment at the Sierra Foothill Research
and Extension Center (SFREC): http://danrrec.ucdavis.edu/sierra_foothill/home_page.html.
Please take a look at the SFREC website, and
if you see any opportunity to do a project at SFREC, please contact me.
It is a unique resource for research, extension and teaching programs
in the Sierra Foothills. Sandy Ogletree will continue to
assemble this newsletter so this should not impact our "occasional and
irregular interval" publication schedule.
~~ Art
Craigmill


DPR
Releases 2004 Pesticide Use Data;
More Nature-friendly Chemicals Gain Favor
The California Department of Pesticide
Regulation has reported a small increase in pounds of pesticides
applied in 2004,
but that included a dramatic rise in the use of some
nature-friendly chemicals.
Commercial pesticide use increased from 175
million pounds in 2003
to180 million pounds in 2004, an increase of less than 3 percent.
More than half of the five million pound
increase in 2004 could be linked to two chemicals that qualify for
organic agriculture
-- sulfur and mineral oils. In addition, "A dramatic increase
occurred in
the use of some newer, reduced-risk pesticides," said DPR analysts.
Meanwhile, use of several classes of highly-toxic chemicals declined,
both in pounds applied and acres treated.
DPR Director Mary Ann Warmerdam said the
statistics were timely.
"They coincide with DPR policy initiatives to emphasize more
sustainable, less toxic pest management for agriculture
and industry,
and in homes and gardens," said Warmerdam. "This is just another
indication that we
are moving in the right direction."
Last year, Warmerdam directed DPR's Pest
Management Advisory
Committee to begin developing a statewide blueprint for integrated pest
management (IPM), a least-toxic approach that stresses more
prevention and
less reliance on chemicals. A diverse workgroup made recommendations to
the committee late last year. DPR expects to move forward on its
IPM blueprint after the pest management committee meets in February,
said Warmerdam.
"The recommendations include more IPM research,
as well as public-private cooperative efforts that offer strong and
positive incentives to industry," said Warmerdam. She also
welcomed a recommendation for renewed support of IPM grant
programs.
DPR produced dozens of successful IPM projects around the state,
until budget
cuts suspended the IPM grants in 2003.
Some details from the 2004 DPR pesticide use summary:
- Pesticide use varies from year to year based on many factors,
including types of crops, economics, acreage planted, and other factors
--
most notably weather. A wet winter in 2004 promoted weed
growth;
then a hot, dry summer encouraged mites and other pests. In addition,
acreage increased for some major crops, and high-value crops
often justify
more intensive pest management.
- As measured by pounds, sulfur was the most-used chemical with 54
million pounds, or about 30 percent of all pounds applied. Sulfur
-- favored by both conventional and organic farmers -- saw
use
increase by nearly 800,000 pounds (1.5 percent) in 2004. Use of mineral
oil,
another chemical that qualifies for organic production, increased
by 2.8
million pounds (44 percent).
- Meanwhile, "A dramatic increase occurred in the use of some
newer, reduced-risk pesticides such as spinosad, acetamiprid,
pyraclostrobin, methoxyfenozide, carfentrazone-ethyl, and
boscalid," DPR analysts reported.
- Spinosad is a relatively new chemical class of insecticides
derived from a natural soil bacterium. It was first discovered by a
vacationing scientist in an abandoned rum distillery in the
Caribbean.
Spinosad use increased by 4,400 pounds and 52,000 acres -- to a total
of more
than 858,000 cumulative acres -- in 2004. Use of insecticide
organophosphate and carbamate chemicals --
compounds of high regulatory concern -- continued to decline. Use
declined by 130,000 pounds (1.6 percent) and by 360,000 acres
treated (5.7
percent) in 2004, compared to the prior year.
- Use of chemicals classified as reproductive toxins declined by
600,000 pounds (2.5 percent), and by cumulative acres treated, 180,000
acres (7.7 percent). The fumigant methyl bromide showed the
largest
decline in pounds -- 295,000 -- or 4 percent.
- Another major fumigant, metam-sodium, decreased by 132,000 pounds
(1 percent) and about 14,000 cumulative acres (10 percent). Use of the
fumigant 1,3-D increased by 1.9 million pounds (28 percent) and
about 7,700 acres (16 percent).
- As in previous years, the most pesticide use occurred in the San
Joaquin Valley, the nation's No. 1 agricultural area. Fresno, Kern,
Tulare, and San Joaquin counties had the highest poundage use.
- Pesticide use is reported as the number of pounds of active
ingredient and the total number of acres treated. Data for pounds
includes
both agricultural and nonagricultural applications; data for
acres
treated are primarily agricultural applications. The number of acres
treated is cumulative; one acre treated three times is counted as
three acres.
For the 2004 Pesticide Use Report Summary and selected "top" data
lists, link to
REF: DPR News Release, January 23, 2006.


Orf
Virus Infection in Humans
New York, Illinois, California, and Tennessee, 2004-2005
Orf virus is a zoonotic parapoxvirus endemic to most
countries in the world and is principally associated with small
ruminants (e.g., sheep and goats). Human orf infections appear as
ulcerative skin lesions after contact with an infected animal or
contaminated fomite. This
report summarizes the epidemiologic and laboratory investigations
of four sporadic cases of human orf infection, emphasizing the temporal
association between human lesions and skin trauma or recent flock
vaccination with live orf vaccine. This zoonotic infection shares
clinical manifestations and exposure risks with other, potentially
life-threatening zoonoses (e.g., cutaneous anthrax) and is likely
under-recognized because of a lack of clinical suspicion and widely
available diagnostics. Barrier precautions and proper hand hygiene are
recommended for the prevention of orf virus infection in humans.
Case 1. On March 1, 2004, a woman aged 51 years from upstate New
York noted an area of erythema approximately 4 mm in diameter on the
middle finger of her right hand. During the next several days, the
lesion evolved into a clear, solitary vesicle with surrounding
erythema. On March 12, she visited her family physician, who prescribed
penicillin and warm water soaks. The patient did not recall any trauma,
including animal bites, although she regularly cared for goats on her
family farm. She reported having bottle-fed a kid goat with a sore on
its mouth approximately 1 week before the appearance of the lesion.
The patient did not improve and, on March 15, she went to a local
hospital. The lesion on her finger had progressed to 2 cm in diameter
with a 3-4 mm central white ring and umbilication. Her examination was
otherwise unremarkable. The patient was treated empirically with
ciprofloxacin
and amoxicillin-clavulanate.
On March 22, after discussion with local veterinarians, she contacted
the New York State Department of Health to inquire about diagnostics
for orf virus infection. Specimens collected on March 15 were forwarded
to CDC and determined to be positive at both genus (Parapoxvirus)
and species (orf virus). By
April 1, the lesion had spontaneously healed without scarring. No other
family members or farm attendants reported similar skin lesions.
Case 2. In May 2004, an adolescent boy aged 16 years was bitten
on the left hand by a healthy-appearing sheep that he was grooming for
a county fair in southwestern Illinois. The sheep had been vaccinated
against orf virus 1 week before the patient was bitten. Three weeks
after he sustained the bite, the patient went to his primary-care
physician with three nonpruritic, painless vesicular lesions on his
left thumb, the largest of which was 1.5 cm in diameter (See Figure
below). The patient reported no constitutional symptoms,
and the rest of his physical examination was unremarkable. CDC
confirmed the diagnosis of orf virus infection. No treatment was
administered, and the lesions healed
spontaneously after 2 months. The sheep was removed from the county
fair once the orf infection was evident, and active case finding failed
to reveal other orf infections in county fair staff or attendees.
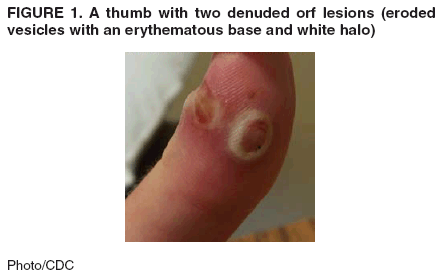
Case 3. On July 28, 2004, a man aged 51 years from Sonoma
County, California, was referred to an infectious diseases physician
because of pruritic, painless vesicles on his left hand. He had onset
of these lesions 10 days after shearing young sheep, which had been
purchased recently at auction and vaccinated with the live orf vaccine.
The patient noted that some of the sheep had ulcers on their oral
mucosa. He also recalled cutting his skin on thistles and burs embedded
in the sheep wool. He reported no constitutional symptoms. His physical
examination was only remarkable for five bullae (vesicles >1
cm in diameter), 1.0-1.5 cm in diameter, on the back of both hands.
Histopathology indicated nonspecific inflammation, but serologic
evaluation revealed parapoxvirus,
consistent with current or recent parapoxvirus infection. All lesions
healed spontaneously within 2 weeks.
Case 4. On May 25, 2005, a girl aged 11 years was taken to
her pediatrician in Nashville, Tennessee, with a 7-mm papulovesicular
lesion on the fourth finger of her left hand. Ten days before this
visit, her family had vaccinated their sheep against orf virus. Five
days before her clinic visit, she had cut the same finger on a lamb
harness. The remainder of her physical examination was unremarkable.
CDC confirmed the presence of orf virus using both genus-
and species-specific primers, and standard PCR assays were negative for
both primer sets. The lesion healed spontaneously within 1 month. No
other family members reported similar lesions to the attending
physician.
Editorial Note:
Although orf virus infection is self-limiting in hosts with normal
immune systems, it can resemble skin lesions associated with
potentially life-threatening zoonotic infections such as tularemia,
cutaneous anthrax, and erysipeloid; therefore, rapid and
definitive diagnosis is critical. Tularemia and erysipeloid are
generally associated with exposure to rabbits or New World sylvan
rodents and swine, respectively. Both orf virus infection and naturally
acquired anthrax in humans can result from exposure to domestic sheep
and goats; thus, exposure history alone (i.e., animal contact) is
insufficient to indicate etiology, necessitating laboratory
evaluation.
Transmission of orf virus to humans occurs after contact with
infected or recently vaccinated animals and/or fomites in conjunction
with skin trauma. Orf virus vaccine strains have been known to cause
outbreaks among sheep, and three of the illnesses described
in this report occurred soon after vaccination of the flock. Veterinary
vaccines for orf virus use nonattenuated, live virus preparations and
are intended to produce controlled infections in flocks.
Recently vaccinated animals pose an occupational risk to humans.
Infections in three of the four cases described in this report were
temporally associated with orf virus vaccination; however, the vaccines
used to inoculate the animals in question were not available for
genetic comparison with patient isolates.
Three of the four cases described in this report were associated
with concurrent skin trauma; orf virus infection is facilitated by skin
trauma, and previous case series have associated skin trauma
with orf virus infection. Trivial injury (e.g., pricks from
thistle) or substantial trauma (e.g., bites) can facilitate
transmission of orf virus. Therefore, barrier protection (e.g.,
nonporous gloves) and hand washing during the care of sheep and goats
is recommended whenever feasible. These measures are especially
important for any person with a compromised immune system or a chronic
skin disorder (e.g., eczema) who has contact with overtly infected
animals. Immunocompromised persons should discuss the risks of handling
orf-infected animals and infection-prevention strategies with their
primary-care physicians.
Human orf virus infection is a common yet preventable consequence of
contact with sheep and goats. Persons who are most likely to be exposed
to orf virus (e.g., farm workers) might be familiar with the infection
and thus might not seek medical attention. As a result, clinicians
might not be familiar with orf virus infections, leading to a delay in
diagnosis and unnecessary antibiotic use. Public health personnel
should be cognizant that orf virus infection is similar in appearance
and risk factors to life-threatening infections such as cutaneous
anthrax and that skin trauma is a predisposing factor to infection. In
addition, immunocompromised patients can have progressive, destructive
lesions requiring medical interventions such as antiviral therapy
and surgical debridement. The relation between vaccination
of sheep and goats for orf virus and subsequent human orf virus
infection should be considered in future public health investigations.
Barrier precautions and proper hand hygiene are recommended for the
prevention of orf virus infection in humans.
REF: MMWR Weekly, January 27, 2006 /
55(03);65-68

Animal Health and
Consumer Protection
Nearly a century ago, farmers had a medicine
chest of products to
"cure" their animals, with names such as Lee's Gizzard Capsules, Liquid
Hog Medicine, and Kow-Kure. The gizzard capsules, made with nicotine,
were advertised to get rid of worms in turkeys. Liquid Hog Medicine,
which contained lye, was for treating diarrhea in pigs. And Kow-Kure,
whose exact ingredients remain a mystery, purported to prevent
miscarriages in cows.
No one knew whether these products were actually
safe or effective, but
all were allowed on the market under the federal drug laws at the time.
Such products went by the wayside as Congress
passed stronger drug
laws, and today, the regulation of animal drugs closely parallels the
regulation of human drugs. Like human drugs, all animal drugs must be
approved by the Food and Drug Administration before being allowed on
the market. The FDA's Center for Veterinary Medicine (CVM) is
responsible for regulating drugs and food additives used for
animals-both food-producing animals and family pets.
To date, the CVM has approved nearly 700 drug
products for use in 97
million cattle, 59 million pigs, 8.8 billion chickens, 272 million
turkeys, 7 million sheep, and millions of other food-producing animals
in the United States. In addition, more than 700 approved drug products
are available to maintain the health of America's 60 million pet dogs,
75 million pet cats, and 5 million horses.
To read the entire article link to:
http://www.fda.gov/
REF: FDA Consumer Magazine, January/February 2006

Death of a Child
After Ingestion of a Metallic Charm
Minnesota, 2006
Lead-based paint remains the most common source
of lead exposure for children aged <6 years. However, one report
determined that 34% of children aged <6 years with lead poisoning in
Los Angeles County had been exposed to items containing lead that had
been brought into the home. These items might include candy, folk and
traditional medications, ceramic dinnerware, and metallic toys and
trinkets. Exposures to some of these items can result in
life-threatening BLLs of >100 µg/dL (elevated
BLLs are >10 µg/dL for children and >25
µg/dL for adults). In 2004, a child in Oregon had a BLL of
123 µg/dL after ingesting a necklace with high lead
content. The same year, the Consumer Product Safety Commission (CPSC)
recalled 150 million pieces of imported metallic toy jewelry sold in
vending machines. Some lead-contaminated items intended for use by
children are manufactured in countries with limited government
regulation of lead in consumer products. With the decline in BLLs in
U.S. children, widespread education of the dangers of lead paint, and
systematic reduction of lead hazards in U.S. housing, acute ingestion
of lead-containing items has become increasingly more common as a
source of life-threatening BLLs.
This
report describes the death of a child from
acute lead poisoning caused by lead encephalopathy after ingestion of a
heart-shaped metallic charm containing lead; the charm had been
attached to a metal bracelet provided as a free gift with the purchase
of shoes manufactured by Reebok International Ltd. On March 23, a
voluntary recall of 300,000 heart-shaped charm bracelets was announced
by CPSC and Reebok (see Figure below). Health-care providers should
consider lead poisoning in young children with increased intracranial
pressure, unexplained and prolonged gastric symptoms, or a history of
mouthing or ingesting nonfood items. Health-care providers also should
warn caregivers against allowing children to mouth any metal objects.
In mid-February 2006, a boy aged 4 years with a
previous medical history of microcephaly and developmental delay was
brought to a hospital pediatric emergency department in Minneapolis,
Minnesota, with a chief complaint of vomiting. Probable viral
gastroenteritis was diagnosed, and the boy was administered
ondansetron, an antiemetic; his parents were encouraged to increase his
fluid intake, and he was released. He returned to the emergency
department 2 days later with intractable vomiting, poor oral intake,
"sore tummy," and listlessness. He was dehydrated and had normal blood
sodium and elevated blood urea nitrogen levels. He received intravenous
fluid replacement and was admitted to the hospital.
The next day, about 10 hours after admission, the
boy became agitated and combative and exhibited possible posturing.
During transport to the radiology department, the boy suffered a
respiratory arrest associated with seizure-type activity. He was
resuscitated and placed on mechanical ventilation. He was administered
a computer tomography (CT) scan of his head and of his chest and
radiographs of his abdomen. The CT scan revealed diffuse cerebral
edema, and the boy underwent emergent ventriculostomy and decompressive
craniotomy. A heart-shaped object was observed on his abdominal
radiographs but it was thought to be a radiopaque temperature probe on
his body. When the radiographs were examined again, the object was
recognized as a foreign body in his stomach, and testing for heavy
metal levels was requested.
The next day, a BLL of 180 µg/dL
was reported; cerebral blood flow studies indicated no flow to the
brain, and the boy met clinical brain death criteria. On the fourth day
of hospitalization, the child was removed from life support and died.
Upon autopsy, a heart-shaped charm imprinted with "Reebok" was removed
from the child's stomach. The mother recognized the object as a charm
that came with a pair of shoes belonging to another child whose home
her son had visited. The mother was not aware that her son had ingested
the charm, and he had no history of ingesting nonfood substances.
Acid digestion testing performed on the ingested
charm determined that the charm consisted of 99.1% lead. CPSC suggests
that tests for leaching be conducted on those items containing more
than 0.06% lead by weight. A charm similar in size and shape to the one
ingested, with Reebok imprinted on it, was obtained by Minneapolis
Department of Regulatory Services staff members at an athletic shoe
store in Minneapolis and tested by the same laboratory using the same
method. Results determined that the charm consisted of 67.0% lead by
weight. The same staff member purchased another look-alike charm with a
pair of athletic shoes from the Reebok Internet site; this charm was
tested by the same Minneapolis laboratory using the same testing method
and determined to contain only 0.07% lead by weight.
In Atlanta, Georgia, CDC staff members purchased
four pairs of athletic shoes of the same brand, including two pairs
with look-alike charm bracelets and two pairs with both charm bracelets
and shoelace charms, from local stores and from the company's Internet
site; they also obtained a promotional charm bracelet from a different
athletic shoe manufacturer. Acid digestion analyses were conducted and
revealed lead contents ranging from 0.004% to 0.044% by weight.
The variation in lead content revealed by the
tests in Minneapolis and Atlanta is consistent with previous test
results for small, inexpensive metallic jewelry. The variations in lead
content of the charms purchased in Atlanta stores and from the
company's Internet site were not as varied as those in Minneapolis,
likely indicating different suppliers or production lots.
As the variation in lead content in these
products indicates, alternatives to lead are available. Restriction or
elimination of nonessential uses of lead in consumer products should be
part of a proactive strategy that prevents exposure to these products
and is preferable to relying on case finding to identify lead exposure
hazards.

REF: MMWR, March
23, 2006 / 55(Dispatch);1-2.

Pesticides
in the Nation’s Streams and
Ground Water
The U.S. Geological Survey has released a report
describing the
occurrence of pesticides in streams and ground water during 1992-2001.
The report concludes that pesticides are typically present throughout
the year in most streams in urban and agricultural areas of the Nation,
but are less common in ground water. The report also
concludes that pesticides are seldom at concentrations likely to affect
humans. However in many streams, particularly those draining urban and
agricultural areas, pesticides were found at concentrations that may
affect aquatic life or fish-eating wildlife.
Dr. Robert Hirsch, Associate Director for Water,
said, "While the
use of pesticides has resulted in a wide range of benefits to control
weeds, insects, and other pests, including increased food production
and reduction of insect-borne disease, their use also raises questions
about possible effects on the environment, including water quality."
Hirsch also commented that "the USGS assessment provides the most
comprehensive national-scale analysis to date of pesticide occurrence
in streams and ground water. Findings show where, when, and why
specific pesticides occur, and yield science-based implications for
assessing and managing pesticides in our water resources."
The USGS findings show strong relations between
the occurrence of
pesticides and their use, and point out that some of the frequently
detected pesticides, including the insecticide diazinon and the
herbicides alachlor and cyanazine, are declining.
USGS has worked closely with the U.S.
Environmental Protection
Agency (EPA) during the 10-year study. EPA uses the data extensively in
their exposure and risk assessments for regulating the use of
pesticides. For example, EPA used USGS data in its risk assessments for
the reevaluation of diazinon, chlorpyrifos, cyanazine and alachlor.
Uses of three of these pesticides (diazinon, chlorpyrifos and
cyanazine) have now been significantly limited, and usage of alachlor
was voluntarily reduced and largely replaced by a registered
alternative.
The USGS report is based on analysis of data
collected from 51 major
river basins and aquifer systems across the Nation from Florida to the
Pacific Northwest and including Hawaii and Alaska, plus a regional
study in the High Plains aquifer system.
Although none of the USGS stream sampling sites
were located at
drinking-water intakes, a screening-level assessment was done by USGS
to provide an initial perspective on the relevance of the pesticide
concentrations to human health. USGS measurements were compared to EPA
drinking-water standards and guidelines. Concentrations of individual
pesticides were almost always lower than the standards and guidelines,
representing less than 10 percent of the sampled stream
sites and about 1 percent of domestic and public-supply wells.
However, pesticides may have substantially
greater effects on
aquatic ecosystems than on humans based on a screening-level comparison
of USGS measurements to water-quality benchmarks for aquatic life and
fish-eating wildlife. More than 80 percent of urban streams and more
than 50 percent of agricultural streams had concentrations in water of
at least one pesticide—mostly those in use during the study period—that
exceeded a water-quality benchmark for aquatic life. Water-quality
benchmarks are estimates of concentrations above which pesticides may
have adverse effects on human health, aquatic life, or fish-eating
wildlife.
Insecticides, particularly diazinon,
chlorpyrifos, and malathion
frequently exceeded aquatic-life benchmarks in urban streams. Most
urban uses of diazinon and chlorpyrifos, such as on lawns and gardens,
have been phased out since 2001 because of use restrictions imposed by
the EPA. The USGS data indicate that concentrations of these pesticides
may have been declining in some urban streams even before
2001—benchmark exceedences in urban streams were least frequent late in
the study. A case study of diazinon shows declining concentrations in
several urban streams in the Northeast during 1998-2004.
In agricultural streams, the pesticides
chlorpyrifos,
azinphos-methyl, p,p’-DDE, and alachlor were among those most often
found at concentrations that may affect aquatic life, with each being
most important in areas where its use on crops is or was greatest.
According to senior author Robert Gilliom, however, "Pesticide use is
constantly changing in response to such factors as regulations and
market forces and findings from this decade-long study need to be
examined in relation to changes in use during and after the study. For
example, levels of the herbicide alachlor declined in streams in the
Corn Belt (generally including Illinois, Indiana, Iowa, Nebraska, and
Ohio, as well as parts of adjoining states) throughout the study period
as its use on corn and soybeans declined, with no levels greater than
its aquatic-life benchmark by the end of the study. In contrast, both
the use and the levels of atrazine, the most heavily used herbicide in
the Corn Belt region, remained relatively high throughout the study
period."
In addition, DDT, dieldrin, and
chlordane—organochlorine pesticide
compounds that were no longer in use when the study began—were
frequently detected in bed sediment and fish in urban and agricultural
areas. Concentrations of these compounds in fish declined following
reductions in their use during the 1960s and elimination of all uses in
the 1970s and 1980s, and continue to slowly decline. Just as notable as
the declines, however, is the finding that these persistent
organochlorine pesticides still occur at levels greater than benchmarks
for aquatic life and fish-eating wildlife in many urban and
agricultural streams across the Nation.
The USGS study also reported that pesticides
seldom occurred
alone—but almost always as complex mixtures. Most stream samples and
about half of the well samples contained two or more pesticides, and
frequently more.
Gilliom explained that "The potential effects of
contaminant
mixtures on people, aquatic life, and fish-eating wildlife are still
poorly understood and most toxicity information, as well as the
water-quality benchmarks used in this study, has been developed for
individual chemicals. The common occurrence of pesticide mixtures,
particularly in streams, means that the total combined toxicity of
pesticides in water, sediment, and fish may be greater than that of any
single pesticide compound that is present. Studies of the effects of
mixtures are still in the early stages, and it may take years for
researchers to attain major advances in understanding the actual
potential for effects. Our results indicate, however, that studies of
mixtures should be a high priority."
REF: United States Geological Survey website,
http://www.usgs.gov/newsroom/article.asp?ID=1450,
3/3/2006

"Top 10 Pesticide
Blunders" Provide Cautionary Tales
The California Department of
Pesticide
Regulation has announced its third annual "Top 10 Pesticide Blunders."
With the best interests of
consumer and worker
safety in mind, DPR
also recapped leading cases from the two previous years:
-- "As a 34-year-old Yolo County motorist moved her
driver's seat
backward, the motion caused an insect fogger stashed underneath the
seat to discharge..."
-- "A 23-year-old San Joaquin County man spotted a fly
on his beer
can, sprayed an insecticide on the can, and later, as he drank from the
can, his lips began to tingle..."
These and the new list of blunders below graphically
demonstrate
what NOT to do as you undertake household and gardening chores or other
work with pesticides this spring. DPR health and safety scientists
say a few simple precautions can prevent most pesticide accidents:
-- Look for the least-toxic solution to pest
problems, indoors and
out.
-- Read all pesticide label directions closely and
follow
directions to the letter.
-- Keep pesticides in their original containers and
out of
children's reach.
Many home pesticide accidents
occur in kitchens and
bathrooms.
Almost half of households with children under age five
have at least one pesticide stored within a child's reach, according
to national
health surveys. Children are especially vulnerable when
adults put
pesticides into drinking containers, such as soda or juice
bottles. Consumer pesticide products with colorful packaging and
attractive scents
may also attract children.
The third annual "blunders" list
had fewer
potential candidates, due to a decline in reports. In recent years, DPR
lost funding to
pursue consumer pesticide illnesses. For example, a DPR
cooperative
project with state poison control centers was suspended,
due to lack of
funds. As resources allow, DPR continues to work with
health agencies to improve detection of non-occupational illnesses.
None of these latest "blunders"
-- compiled from
DPR's Pesticide Illness Surveillance Program -- resulted in death,
although most
victims required medical treatment. (State privacy law
protects their identities.)
In no particular order, the "top 10" are:
1. As a San Diego County man prepared to spray ants
with
insecticide, he failed to notice the aerosol can faced the wrong
way. He sprayed himself in the face, developed respiratory
symptoms, and sought
medical attention the next morning.
2. In Los Angeles County, a woman sprayed an
aerosol insecticide
under her kitchen sink to kill roaches. To get a better
shot, she stuck
her head inside the cabinet and then inhaled fumes. Her
lungs began to
burn and she sought medical attention.
3. An Orange County resident set off two "bug
bombs" and left his house. He returned 90 minutes later, opened the
windows, and
remained inside. He developed heart symptoms and went to a
hospital, where
he suffered a stroke.
4. Another Los Angeles resident who sprayed her
kitchen to kill
flies drank from a glass of water that sat uncovered in
the same room
while she sprayed. A runny nose, headache, and chest
tightness prompted
her to seek medical aid.
5. In Orange County, a dog owner with asthma hugged
her one-pound
puppy shortly after it received a liquid flea control
treatment from the woman's veterinarian. It was later determined that
the puppy was treated with a dosage meant for larger dogs. The
owner experienced shortness of breath, blurry vision, and other
symptoms. The puppy
also apparently suffered ill effects.
6. A San Diego receptionist sprayed an insecticide
around doors in
her office for spiders. She got the pesticide on her
hands so she
rubbed them together. She later rubbed her eyes. Her hands
and eyes began
to itch, so she sought medical attention.
7. A San Bernardino truck driver prepared to
disinfect his tires
with a hose-mounted sprayer. When he pulled on the hose,
it knocked the attached disinfectant bottle off. The bottle hit
the ground and disinfectant splashed into his face and eyes.
8. A Los Angeles County worker prepared to mop a
kitchen floor
when she noticed she was almost out of the usual cleaning
product. She mixed bleach with the cleaning product, which created
fumes. She
developed respiratory symptoms and sought medical attention.
9. At a San Bernardino County fast-food outlet, a
customer at the drive-through window bought iced tea and noticed a
foul taste,
followed by a burning throat and nasal passages. The cup
apparently
contained some sanitizer from an improperly rinsed tea
machine. (Similar case reported in Los Angeles County.)
10. A Marin County lifeguard mistakenly added
muriatic acid to a chlorine tank. He inhaled the resulting fumes and
developed
symptoms. His mother saw him coughing and took him for
medical aid.
REF: Department of Pesticide
Regulation; News, April 18, 2006, www.cdpr.ca.gov/

FDA is committed to ensuring the safety of food
and beverages consumed by Americans and providing timely and factual
information when safety questions are raised. We are issuing this
statement today to better describe the steps FDA is taking in response
to reports that benzene has been found in some soft drinks.
Benzene, a carcinogen, is found in the
environment from natural and man-made sources. In November 2005, FDA
received reports that benzene had been detected at low levels in some
soft drinks containing benzoate salts (an antimicrobial agent) and
ascorbic acid (Vitamin C), particularly under certain conditions of
storage, shelf life and handling.
FDA's Center for Food Safety and Applied
Nutrition (CFSAN) initiated a survey of benzene levels in soft drinks
following receipt of the November 2005 reports. This survey indicates
that the vast majority of beverages sampled (including those containing
both benzoate salts and ascorbic acid) contain
either no detectable benzene levels or are well below the 5 parts per
billion (ppb) U.S. water standard. The results of this survey,
which will be released in the near future, indicate that the levels of
benzene in these beverages do not
pose a safety concern.
FDA's Total Diet Study (TDS) results from 1995 to
2001, indicated benzene levels in soft drinks that were well above and
inconsistent with CFSAN's more recent survey results. The TDS results
were also well above and inconsistent with levels reported in previous
and current peer-reviewed literature and with hundreds of recent
domestic and international government and beverage industry results. We
are working to determine the source of the differences. As with any
data that appear to be inconsistent, FDA believes it is important to
closely examine the reasons for such differences.
The TDS is an ongoing FDA program that determines
levels of various contaminants and nutrients in a wide variety of
foods. The analytical procedures used in the TDS are designed to detect
multiple pesticide residues, industrial chemicals, and toxic and
nutrient elements in many foods, not just benzene in beverages. Ongoing
investigations into the analytical method used by the TDS suggest that
elevated benzene levels can be formed by the procedures used to analyze
beverage samples. This raises major concerns about the reliability of
the TDS data for benzene in beverages and could explain why these data
indicate higher levels of benzene than the levels reported in the more
recent surveys by CFSAN and others, as noted above. We are continuing
our investigation of the TDS data for benzene, and will make the
results available when the investigation is complete.
FDA is also continuing to follow up with
companies to ensure that processing conditions are established that
will ensure that benzene formation is avoided or minimized.
FDA believes that the results of CFSAN's recent
survey indicate that the levels of benzene found in soft drinks do
not
pose a safety concern.
REF: FDA News Digest, April 17, 2006, FDA
website

Comprehensive
dietary supplement reports issued
The National Institutes of Health has drafted a
"state-of-the-science" report about whether multivitamin/mineral
supplements (MVMs) and certain single nutrient supplements can prevent
chronic disease. The conclusions expressed by the report's authors
include:
**More than half of American adults take MVMs with the belief that
they will feel better, have greater energy, improve health, and/or
prevent and treat disease.
**Compared with nonusers, supplements takers tend to have a better
diet, less need for supplements, and more risk of exceeding the safe
upper limit (UL) of some nutrients.
**There is insufficient evidence to recommend either for or
against the use of MVMs by the American public to prevent chronic
disease.
**Few high-quality studies have addressed whether one or a few
nutrients can prevent chronic disease in American adults, and only a
few such studies have yielded positive results.
**With few exceptions, neither beta-carotene nor vitamin E had
benefits for preventing cancer, cardiovascular disease, cataract, and
age-related macular degeneration. Beta-carotene supplementation
increased lung cancer risk in smokers and persons exposed to asbestos.
**Folic acid alone or combined with vitamin B12 and/or vitamin B6
had no significant effect on cognitive function.
**Selenium may confer benefit for cancer prevention but not
cardiovascular disease prevention.
**Calcium may prevent bone mineral density loss in postmenopausal
women and may reduce vertebral fractures, but not non-vertebral
fractures. The evidence suggests dose-dependent benefits of vitamin D
with or without calcium for retaining bone mineral density and
preventing hip and other nonvertebral fractures.
**The FDA lacks the resources to collect adequate data and lacks
the legal authority to safely regulate the dosage of individual
ingredients.
**Additional research and a mandatory adverse-event reporting
system are needed for dietary supplements.
 TOXICOLOGY
TIDBITS
TOXICOLOGY
TIDBITS 
Surface Microbial Contamination
on Fresh Produce
Much effort has been focused on sanitation of fresh
produce at the
commercial level; however, few options are available to the consumer.
The purpose of this study was to determine the efficacy of different
cleaning methods in reducing bacterial contamination on fresh produce
in a home setting. Lettuce, broccoli, apples, and tomatoes were
inoculated with Listeria innocua and then subjected to combinations of
the following cleaning procedures: (i) soak for 2 min in tap water,
Veggie Wash solution, 5% vinegar solution, or 13% lemon solution and
(ii) rinse under running tap water, rinse and rub under running tap
water, brush under running tap water, or wipe with wet/dry paper towel.
Presoaking in water before rinsing significantly reduced bacteria in
apples, tomatoes, and lettuce, but not in broccoli. Wiping apples and
tomatoes with wet or dry paper towels showed lower bacterial reductions
compared with soaking and rinsing procedures. Blossom ends of apples
were more contaminated than the surface after soaking and rinsing;
similar results were observed between the flower section and stem of
broccoli. Reductions of L. innocua in both tomatoes and apples were
more than in lettuce and broccoli when subjected to the same washing
procedures. Reductions of
surface contamination of lettuce after soaking in lemon or vinegar
solutions were not significantly different from lettuce
soaking in cold tap water. Therefore, educators and extension workers
might consider it appropriate to instruct consumers to rub or brush
fresh produce under cold running tap water before consumption. (Journal of Food Protection, Vol.
69, No. 2, pp. 330–334.)
REF: FSnet Feb. 15/06 -- II
News from the
California Department of Pesticide
Regulation
SCHOOL IPM PAGES ADD INFO
The School IPM Web page now
offers a summary of
Assembly Bill 405,
which took effect on January 1, and a list of list of pesticides
prohibited from use in schools under the law. Also new
information
on gopher and mold control strategies for schools. www.cdpr.ca.gov/cfdocs/apps/schoolipm/main.cfm
REF: Department of Pesticide Regulation
News, February 7, 2006.


Health Canada
issues chaparral warning
Health Canada is warning consumers not to
ingest the herb
chaparral in the form of loose leaves, teas, capsules or bulk herbal
products because of the risk of liver and kidney problems. Chaparral
refers to three plant species: Larrea tridentata, Larrea divaricata and
Larrea mexicana, which may also be called creosote bush, greasewood, or
hediondilla. The shrub grows in the Western United States and parts of
Mexico and is used traditionally by the indigenous people of these
regions to treat such conditions as arthritis, cancer, tuberculosis,
bowel cramps, diarrhea, venereal disease, colds and bronchitis. No
chaparral-containing products are currently approved by Health Canada
for any use. [Health Canada News release, Dec 21, 2005]
http://www.inspection.gc.ca/english/corpaffr/recarapp/2005/20051222e.shtml
 Petting zoo visits
linked to
fever and diarrhea in kids
Petting zoo visits
linked to
fever and diarrhea in kids
Petting zoos at agricultural fairs, festivals, and
zoos let kids
interact with animals like goats, cows, sheep, and llamas. But few
regulations exist to ensure that petting zoo animals are free of
disease, and several nationwide outbreaks of E. coli infection have
been linked to these attractions, say researchers from the Centers for
Disease Control and Prevention (CDC) in Atlanta, Georgia.
During 2004 to 2005, petting zoo problems appeared in three states:
North Carolina: In October
2004, about 800,000 people attended the
North Carolina State Fair, which provided two petting zoos for kids. At
the end of October, the state health department received reports of
hemolytic uremic syndrome (HUS, a condition that causes severe anemia
and kidney damage) in three children who had visited the petting zoo.
Local health departments later reported 108 cases of diarrhea (most in
children) close to the time of the state fair, and 78% of the ill
people had visited the state fair petting zoo. Almost 20% of them
needed hospitalization for their illness, which included symptoms such
as bloody diarrhea and fever. Samples taken from the fairgrounds showed
that one of the two petting zoos was contaminated with E. coli,
bacteria found in the feces (bowel movements) of people and animals
that can make both kids and adults sick and is linked to HUS. Illness
in kids was associated with:
- touching or stepping in manure
- falling or sitting on the ground by the petting zoo
- thumb-sucking or using a pacifier or sippy cup while in the
petting zoo
Although having parents who were aware that animals can cause disease
helped to protect kids from illness, using alcohol-based hand
sanitizers didn't help reduce
E. coli
infection.
Florida: In March 2005, 63
people developed E. coli infections (and
seven people developed HUS); most cases developed in children after
they attended fairs and festivals in Florida that contained a farm
animal petting zoo. At least half of those who became sick had touched
at least one cow, sheep, or goat; stool (poop) samples from the animals
and humans tested positive for E. coli. Having indirect animal contact
(such as touching sawdust or shavings or visibly soiled clothes or
shoes) was also associated with infection, which caused symptoms such
as diarrhea, vomiting, abdominal cramps, and fever.
Arizona: In July 2005, the
Arizona Department of Health Services
received reports of two children who had been hospitalized for E. coli
infection. Both kids had visited an Arizona zoo that contained a
petting area. Although one child had directly touched the animals, the
other had not - but both children played in an area right next to and
downhill from the petting zoo facility. Stool samples from some of the
petting zoo animals tested positive for E. coli, and health experts
suspect that the play area close to the petting zoo became contaminated
via drainage.
What This Means to You: The
findings in this CDC report suggest that
parents should be cautious about taking kids to petting zoos. Often,
petting zoos aren't required to check for contamination, and kids may
get sick even if they clean up afterward with hand sanitizers. If you
do choose to visit a petting zoo with your child:
- Wash your child's hands well with soap and water immediately
after
coming in contact with the animals. All family members in the zoo area
should wash their hands right away, too.
- Avoid playing in play areas or grass that's right next to or
downhill
from the zoo.
- Don't let your child use a pacifier, sippy cup, or other item
that is
placed in the mouth while in the petting zoo. Don't allow thumb-sucking
or nail-biting while there, either.
- Don't lean against railings, fence posts, or other stationary
objects
by the petting zoo.
- Watch out for piles of manure or sawdust that may be contaminated
with
the animals' feces.
- If your child has diarrhea, belly pain, vomiting, or fever and
you've
visited a petting zoo recently, talk to your child's doctor.
Source: Morbidity and Mortality Weekly Report, December 23, 2005.
February 2006, Kids Health, http://www.kidshealth.com/research/petting_zoo.html
REF: FSnet, February 23,
2006


Cows'
ability to break down perchlorate documented
Dairy cows can break down up to 80 percent of
perchlorate that they
ingest, according to new research about this chemical (
Proceedings of
the National Academy of Sciences, volume 102, pages
16152-16157).
The findings suggest that this natural "filtering" process may occur in
the rumen, the second of four compartments in a cow's complex stomach.
Perchlorate, which exists naturally in the
environment, has shown up at
very low levels in some milk. In this research, levels in the milk of
cows given various doses of the compound increased slightly as the
dosage increased. But the levels did not rise in direct proportion to
the increased dosage, according to the ARS scientists at Beltsville,
Md., who performed the study.
Work by others has already shown that perchlorate
does not accumulate
in bovine tissue. (
February 22, 2006, ARS Food and Nutrition
Briefs, http://www.ars.usda.gov/is/np/fnrb/fnrb0106.htm)
REF: AnimalNet Feb. 23/06

LactMed: A New NLM Database on Drugs
and Lactation
LactMed, a free online database with
information on
drugs and lactation, is one of the newest additions to the National
Library of Medicine's TOXNET system, a Web-based collection of
resources covering toxicology, chemical safety, and environmental
health.
LactMed may be searched at http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT
Geared to the healthcare practitioner and nursing
mother, LactMed
contains over 450 drug records. It includes information such as
maternal levels in breast milk, infant levels in blood, potential
effects in breastfeeding infants and on lactation itself, the American
Academy of Pediatrics category indicating the level of compatibility of
the drug with breastfeeding, and alternate drugs to consider.
References are included, as is nomenclature information, such as the
drug's Chemical Abstract Service's (CAS) Registry number and its broad
drug class.
LactMed was developed by a pharmacist who is an
expert in this subject.
Three other recognized authorities serve as the database's scientific
review panel. Ancillary resources, such as a glossary of terms related
to drugs and lactation, and breastfeeding links are also offered.
LactMed can be searched together with TOXNET's other
databases in a
multi-database environment, to obtain other relevant information about
drugs. As a work in progress, LactMed will continue to expand with
additional drugs and be enhanced with other substances, such as
industrial chemicals and radiation.
REF: TOXNET

New telephone line
for antimicrobials
The National Pesticide Information Center (NPIC) is
now taking inquiries, via their telephone helpline, 1-800-858-7378,
and web-based services, http://npic.orst.edu/,
regarding antimicrobial pesticides and pesticide products. NPIC is a
toll-free telephone service that provides objective, science-based
information about a wide variety of pesticide-related subjects. The
service is available daily, 6:30 a.m.- 4:30 p.m. (PT).
REF: Pesticide Notes, 25(3), 2006.

Genistein & Soy Formula
Expert Panel Reports
The Center for the Evaluation of Risks
to Human Reproduction (CERHR) announces the availability of the
Genistein & Soy Formula Expert Panel Reports and requests public
comment.
The reports are available on the CERHR website (http://cerhr.niehs.nih.gov).
Written public comments on these reports should be received by July
5, 2006. You may provide input to the NTP at: http://ntp.niehs.nih.gov/go/27902
REF:
http://ntp.niehs.nih.gov

FDA rejects green
tea/cardiovascular health claim
The FDA has concluded that there is no credible
scientific evidence that drinking green tea or green tea extract reduce
the risk of heart disease. In rejecting a petition that sought to allow
tea labels to make that claim, the agency said its review included 105
articles publications submitted with the petition but found "no
credible evidence" to support the requested claims. [Schneeman B.
Letter to Stanley F. Tarka, M.D. May 9, 2006]
http://www.cfsan.fda.gov/~dms/qhcgtea2.html

State
Health Officer Cautions Californians About Ticks and Tick-Borne Diseases
As warmer spring temperatures attract Californians
to outdoor activities, people must take precautions to prevent tick
bites because some ticks carry germs that cause disease, including Lyme
disease, State Public Health Officer Dr. Mark Horton advised today.
"Californians should take measures to reduce
their exposure to ticks when they venture outdoors to work in their
yards and participate in recreational activities, including hiking and
camping," Horton said.
Ticks are small, insect-like creatures that are
often found in naturally vegetated areas throughout California. They
prefer cool, moist environments, shaded grasses, shrubs and leaf
litter. Ticks attach to animals and feed on their blood over several
days.

Individuals may become infected with the bacteria
that cause Lyme disease when they are bitten by an infected western
black-legged tick, the only tick that transmits Lyme disease. The
smaller, immature form of the tick known as a "nymph" is most active
during the spring and early summer months. Roughly the size of a
poppy seed, nymphs are found on logs, tree trunks, fallen branches or
tree limbs and among the damp leaves that accumulate under trees.
Nymphs may attach to people as they gather or sit on logs or walk
through leaf litter. Because nymphs are so small, people may not
notice if one attaches to them.
Early symptoms of Lyme disease often include a
spreading rash, which is usually accompanied by flu-like symptoms, such
as fever and body aches. Prompt treatment with antibiotics can
cure the disease, particularly when it is diagnosed early. If
left untreated, symptoms can progress into arthritis, heart ailments or
nervous system disorders.
Ticks in California can carry other germs that
cause diseases in humans, such as anaplasmosis, ehrlichiosis, Rocky
Mountain spotted fever and babesiosis. The first line of defense
against tick-borne diseases is taking proper personal protective
measures to avoid tick bites. Horton offered the following steps to
reduce exposure to tick bites:
- Avoid areas where ticks live, such as trail margins, brushy and
grassy areas, leaf litter in forests with oak and other hardwood
trees. Stay on trails and avoid contact with logs, tree trunks
and fallen branches or tree limbs in forests.
- When in areas where ticks can be found:
- Wear light-colored clothing so ticks can easily be seen.
- Wear long pants and long-sleeved shirts. Tuck pant legs into
boots or socks and tuck shirts into pants.
- Use a repellent registered for use against ticks.
Repellents with DEET are effective and can be applied to the skin.
Repellents with permethrin are applied to clothing only. Always follow
directions on the container and be especially careful when applying to
children.
- Inspect yourself frequently for ticks while in tick habitat
3. When out of tick-infested areas:
- Conduct a check of your entire body, especially the hairline,
armpit, back of knees and groin, each day for up to three days after
returning from tick habitat. An additional tick check two or three days
after exposure may reveal an engorged tick or a tick bite reaction
that may not have been noticeable before.
- Parents should inspect their children, especially on the scalp
and hairline, after activities in tick-infested areas.
Individuals who discover a tick attached to their
body should remove it as soon as possible to reduce the possibility of
infection. The sooner a tick is removed, the less likely you are
to get sick from an infected tick bite. The tick should be removed by
grasping it with fine-pointed tweezers and pulling it gently, but
firmly, straight out. Insecticides, lighted matches or gasoline
are ineffective and should not be used to remove ticks.
Individuals are advised to wash their hands and apply antiseptic to the
affected area. Individuals who develop a rash, fever or other symptoms
within two to four weeks after being bitten by a tick should consult
their physician immediately.

Additional information is available on the
California Department of Health Services’ Web site at http://www.dhs.ca.gov/ps/dcdc/disb/disbindex.htm
or by calling (916) 552-9730.
REF: CDHS News Release, May 15, 2006.


Youth Tobacco
Surveillance United States, 2001--2002
The National Youth Tobacco Survey (NYTS) and state youth tobacco
surveys (YTS) were developed to provide states with data to support the
design, implementation, and evaluation of comprehensive tobacco-control
programs. This
report summarizes data from the 2002 NYTS and the 2001 and 2002
YTS. Current use of any tobacco product ranged from 13.3% among middle
school students to 28.2% among high school students. Exposure to
secondhand smoke (i.e., environmental tobacco smoke) was high. Media
and advertising influence was also noted. Health and education
officials use YTS and NYTS data to plan, evaluate, and
improve national and state programs to prevent and control youth
tobacco use. States can use these data in presentations to their state
legislators to demonstrate the need for funding comprehensive tobacco
control programs including tobacco cessation and prevention programs
for youth.
REF: MMWR, May 19, 2006.


FDA/EPA
Advisory on Seafood Consumption Still Current
In response to recent inquiries about the FDA/EPA
consumer advisory, “What You Need to Know About Mercury in Fish and
Shellfish,” FDA and EPA want to assure consumers that the advice
contained in the 2004 advisory remains current and that FDA and EPA
stand behind it. The advisory’s recommendations are specific to women
who might become pregnant, women who are pregnant, nursing mothers, and
young children.
Fish and shellfish are an important part of a
healthy diet and can contribute to heart health and children’s proper
growth and development. Because of their many healthy benefits we
recommend that women and young children include them as a regular part
of their diet. However, nearly all fish and shellfish contain traces of
mercury.
By following 3 recommendations for selecting and
eating fish or shellfish, women and young children will receive the
benefits of eating fish and shellfish and be confident that they have
reduced their exposure to the harmful effects of mercury.
- Do not eat Shark, Swordfish, King Mackerel, or Tilefish because
they contain high levels of mercury.
- Eat up to 12 ounces (2 average meals) a week of a variety of fish
and shellfish that are lower in mercury.
- Five of the most commonly eaten fish that are low in
mercury are shrimp, canned light tuna, salmon, pollock, and catfish.
- Another commonly eaten fish, albacore (“white”) tuna has
more mercury than canned light tuna. So, when choosing your two meals
of fish and shellfish, you may eat up to 6 ounces (one average meal) of
albacore tuna per week.
- Check local advisories about the safety of fish caught
by family and friends in your local lakes, rivers, and coastal areas.
If no advice is available eat up to 6 ounces (one average meal) per
week of fish you catch from local waters, but don’t consume any other
fish during that week.
Follow these same recommendations when feeding fish
and shellfish to your young children but serve smaller portions.
FDA continues to test fish and shellfish for
mercury. Should there be a significant change in the underlying science
regarding the risks from methylmercury or the benefits from fish, FDA
and EPA will update the advisory to ensure that the public is informed
when making choices about the amounts and types of fish to eat.
The complete 2004 FDA/EPA advisory, “What You Need
to Know About Mercury in Fish and Shellfish,” can be found at
www.cfsan.fda.gov/~dms/admehg3.html.
REF: FDA
website, June 6, 2006

Mold Prevention Strategies and Possible
Health Effects in the Aftermath of Hurricanes and Major Floods
Summary
Extensive water damage after major hurricanes and
floods increases the likelihood of mold contamination in buildings.
This
report provides information on how to limit exposure to mold and
how to identify and prevent mold-related health effects. Where
uncertainties in scientific knowledge exist, practical applications
designed to be protective of a person's health are presented. Evidence
is included about assessing exposure, clean-up and prevention, personal
protective equipment, health effects, and public health strategies and
recommendations. The recommendations assume that, in the aftermath of
major hurricanes or floods, buildings wet for >48 hours will
generally support visible and extensive mold growth and should be
remediated, and excessive exposure to mold-contaminated materials can
cause adverse health effects in susceptible persons regardless of the
type of mold or the extent of contamination.
For the majority of persons, undisturbed mold is not
a substantial health hazard. Mold is a greater hazard for persons with
conditions such as impaired host defenses or mold allergies. To prevent
exposure that could result in adverse health effects from disturbed
mold, persons should 1) avoid areas where mold contamination is
obvious; 2) use environmental controls; 3) use personal protective
equipment; and 4) keep hands, skin, and clothing clean and free from
mold-contaminated dust.
Clinical evaluation of suspected mold-related
illness should follow conventional clinical guidelines. In addition, in
the aftermath of extensive flooding, health-care providers should be
watchful for unusual mold-related diseases. The development of a public
health surveillance strategy among persons repopulating areas after
extensive flooding is recommended to assess potential health effects
and the effectiveness of prevention efforts. Such a surveillance
program will help CDC and state and local public health officials
refine the guidelines for exposure avoidance, personal protection, and
clean-up and assist health departments to identify unrecognized hazards.
REF: MMWR,
Recommendations and Reports, June 9, 2006 /
55(RR08);1-27.

 Veterinary Notes
Veterinary Notes 
News from the UC Davis California
Animal Health and Food Safety
Laboratory System (CAHFS)
Cattle:
A group of cattle that were moved to a new pasture experienced sudden
death in nine head, three to four days after the move. Rumen content
from one of the animals was positive for lupanine, suggestive of
lupine toxicosis. Ingestion of
toxic amounts of this plant (especially seeds) can lead to a neurotoxic
syndrome characterized by muscle tremors, labored breathing,
convulsions, coma and death. Cattle were moved off the pasture, and no
further deaths occurred.
Horses:
Two horses ingested a total of approximately six pounds of rat bait
pellets containing
diphacinone.
Serum collected from both horses approximately 24 hours after exposure
was positive by HPLC. Diphacinone is a second-generation, long-acting
anticoagulant rodenticide that interferes with normal blood clotting as
a result of reduced concentrations of clotting factors II, VII, IX, and
X. The oral lethal dose for diphacinone in horses has not been reported
in the literature. Both horses were monitored carefully, received
vitamin K1 for 21 days and recovered completely.
Goats:
Lupine poisoning was diagnosed in a group of goats that were moved to a
new field. Five goats died within four hours of being moved. Pathology
findings in three goats included acsites in all and myocardial
degeneration in two. No cardiotoxic compounds were detected in the
rumen contents but alkaloids sparteine and lupanine were detected.
These compounds are consistent with ingestion of lupine. Toxic amounts
of this plant (especially seeds) in goats can lead to neurologic signs.
The livers of all three goats had very low copper concentrations. The
copper deficient state of these goats may have made them more
susceptible to toxins to which goats are normally resistant.
Sheep:
Eleven sheep
died acutely
shortly after being turned out on a harvested broccoli field following
a period of above-average rain and plant regrowth. No lesions were
noted. Nitrate was detected in the aqueous humor fluid at 134 ppm
(toxic >25 ppm), which is indicative of
nitrate intoxication. Clinical signs
of nitrate/nitrite intoxication include salivation, diarrhea, tremors,
ataxia, tachycardia and seizures. Death often occurs within six to 24
hours of exposure.
Llamas:
Oleander poisoning resulted in
a two-day history of depression, lethargy, anorexia, atazia, tremors,
and recumbency in a 5-year-old llama. The nursing baby (cria) remained
healthy. Necropsy revealed
myocardial
degeneration. Stomach content contained plant material that
resembled parts of oleander (Nerium oleander). All parts of the plant
oleander, dried and fresh, are highly toxic.
REF: CAHFS Lab Notes, Winter 2006.


FDA Prohibits Extra-Label Use of
Adamantine and Neuraminidase
The Food and Drug Administration (FDA) is
issuing an order prohibiting the extralabel use of anti-influenza
adamantane and neuraminidase inhibitor drugs in chickens, turkeys, and
ducks. We are issuing this order based on evidence that extralabel use
of these antiinfluenza drugs in chickens, turkeys, and ducks will
likely cause an adverse event in humans.
This rule becomes effective June 20, 2006.
REF: Federal Register, Vol. 71, No. 55 / Wednesday, March 22,
2006

International Workshop on
Minor Use and Minor Species: A Global Perspective
The proceedings of this
Workshop that was
held on October 7, 2004 are available online at http://www.fda.gov/cvm/.
This workshop was jointly sponsored
by the Food and
Drug Administration’s, Center for Veterinary Medicine and the US
Department of Agriculture’s National Research Support Project No. 7, or
NRSP-7. The purpose of the workshop was to provide a global
perspective on drug needs and drug approvals for minor species and
minor uses.


Ask CVM
(The Center for Veterinary Medicine)
- Are mail order pet medications
the same as those I get directly from the veterinarian?
- I need a drug to treat my pet
that you can’t buy in the United States, but it is available overseas.
How can I get permission to import that drug?
- I found the same drug my
veterinarian sells me, but for a much lower price in another country,
and I can order it online.
- Do I need permission from FDA
to import that drug?
- Do I contact CVM if I have a
concern about my vet, such as his treating my dog with drugs not
approved for dogs, or the fact that he makes me buy drugs from him
because he won’t write a prescription the way my doctor will?
- Who in FDA should I notify if
I think there is something wrong with my dog’s commercially made pet
food?
- I’ve seen several food
additive products that are supposed to make my dog feel better. Does
CVM regulate these products?
- I read in a magazine that I
can contact CVM for free health care advice about my pet. Who do I talk
to?
For answers to these questions link
to: FDA
Veterinarian.
REF: FDA Veterinarian, November/December
2005.
!! CLICK ON THE PIG !!



Introduction
DPR Releases 2004 Pesticide Use Data
Orf Virus Infection in Humans
Animal Health and Consumer Protection
Death of a Child After Ingestion of a Metallic Charm
Pesticides in the Nation’s Streams and Ground Water
"Top 10 Pesticide Blunders" provide cautionary tales
Benzene in Soft Drinks
Comprehensive Dietary Supplement Reports Issued
Health Canada Issues Chaparral Warning
Petting Zoo Visits Linked to Fever and Diarrhea in Kids
Cows' Ability to Break Down Perchlorate Documented
LactMed: A New NLM Database on Drugs and Lactation
New telephone line for antimicrobials
Genistein & Soy Formula Expert Panel Reports
FDA rejects green tea/cardiovascular health claim
State Health Officer Cautions Californians About Ticks and Tick-Borne Diseases
FDA/EPA Advisory on Seafood Consumption Still Current
Mold Prevention Strategies and Possible Health Effects in the Aftermath of Hurricanes and Major Floods














































 Veterinary Notes
Veterinary Notes 






