COOPERATIVE EXTENSION
UNIVERSITY OF CALIFORNIA
ENVIRONMENTAL TOXICOLOGY NEWSLETTER
"Published Occasionally at Irregular Intervals"
Vol. 17, No. 3, SEPTEMBER 1997
In This Issue
Introduction
During the last couple of months there has been a major development in the emerging story of "environmental hormones." Last year, a study published in Science found an incredible in vitro synergistic effect between certain "environmental estrogens" at very low levels. (A synergistic effect is one in which the results of two separate exposures result in a far stronger effect than would occur if they simply caused an "additive effect.") The finding that such very low concentrations of these mixtures would produce an effect a thousand times stronger than expected was shocking. The abstract of the initial report stated:
"Combinations of two weak environmental estrogens, such as dieldrin, endosulfan, or toxaphene, were 1000 times as potent in the HER (human estrogen receptor)-mediated transactivation as any chemical alone.... This synergistic interaction of chemical mixtures with the estrogen receptor may have profound environmental implications." (Science 272:1489-1492, 7 June, 1996.)
In late July of this year, the authors formally withdrew the paper in a letter published in Science, stating that they had been unable to replicate the findings, and that other laboratories had been unable to do so as well. They also urged that this be taken into consideration because their results had been used widely. The withdrawal letter states:
"Also, since our publication in Science, others have been unable to reproduce the results we reported. Meanwhile, people in many walks of life have, on their own, put great weight on this report as the basis for much discussion, thought, and even public policy.
Whatever merit this publication contained, and despite the enthusiasm it generated, it is clear that any conclusions drawn from this paper must be suspended until such time, if ever, the data can be substantiated.
None (of the experiments we have conducted to explain the synergy) have provided a satisfactory mechanism to explain our earlier findings. Taken together, it seems evident that there must have been a fundamental flaw in the design of our original experiment." (Science 277:462-3, 25 July, 1997.)
They acted responsibly as scientists to retract their unrepeatable results.
The intent of my introduction and review of this situation is not to dismiss the issue (hormonal activity of environmental contaminants) which was unintentionally "punctuated" by this withdrawn paper. Certainly in the face of an imminent public health hazard, swift action must be taken. My intent is to ask the question in relation to less imminent hazards; "how quickly should we react legislatively or through regulations to controversial findings?" In addition, should "scientific results" be "codified" in laws and regulations which are difficult to change, and if so, how quickly? Scientific results will always be open to change because it is in the nature of science to change our knowledge and understanding. These are difficult public policy questions, because most of us feel that public policy should be based on sound scientific principles whenever possible. I don’t have answers to these questions, but they are the questions which must be addressed in the realm of public policy and public decision making.
Art Craigmill
Tulane University Lab to Retract Study that Reported Dramatic Synergism in Weakly Estrogenic Chemicals
In the July 25 issue of Science, John McLachlan will retract his laboratory’s well-publicized study that found mixtures of weak estrogens could be significantly more powerful when combined than when tested individually.
McLachlan’s retraction will lay to rest an international debate that ensued after Tulane published a study that found mixtures of weak estrogens including two pesticides, dieldren and toxaphene, could be up to 1,600 times more powerful in combination. That study appeared in the June 7, 1996, issue of Science. McLachlan directs the Tulane/Xavier Center for Bioenvironmental Research in New Orleans.
Tulane plans to retract its study because neither the university nor other laboratories can reproduce the results. Tulane’s research captured attention worldwide. The finding offered a possible explanation of how low levels of chemicals might be causing increased breast and testicular cancers along with other maladies people have reported and which some scientists say may be caused by endocrine disruptors -- chemicals that mimic, block or alter hormonal activity.
If the university’s findings had been replicated, EPA could have dramatically reduced pesticide tolerances. At several public forums on endocrine disruptors, Penelope Fenner-Crisp, then deputy director of EPA’s Office of Pesticide Programs, said that if other laboratories reproduced Tulane’s results, EPA likely would have to change the way it assessed risks of endocrine disruptors. EPA currently assumes chemicals act in an additive manner, meaning a mixture’s toxicity can be predicted by summing the potency of the individual chemicals that make up the combination. Occupational exposure limits and other federal standards also could have been affected if Tulane’s results had been repeated.
The Chemical Industry Institute of Toxicology, Duke University Medical School, the National Institute of Environmental Health Sciences and Texas A&M University jointly tried -- and were unable -- to replicate Tulane’s study. The four laboratories’ research was published as a "technical comment" in the January 17 issue of Science; the full study also was published in the April issue of Endocrinology.
Zeneca Central Toxicology Laboratory in Cheshire, England, also was unable to replicate the results. A letter on that study was published in the February 6 issue of Nature.
Three hypotheses explored
McLachlan’s laboratory has been exploring three hypotheses that might explain the synergistic results in his laboratory’s original study. Using a yeast assay, Tulane tried to see if the number of receptors affected the outcome. The synergistic effect, McLachlan speculated, might come into play when chemicals are fighting for a few receptors.
The laboratory also tried to determine whether the structures of the estrogen receptors caused the synergism. Estrogen receptors may be single (monomer) or paired (dimer).
Finally, Tulane tried to determine whether the protein, called a transporter mechanism, that brings chemicals into cells might explain the disparate results. The research team hypothesized that some chemicals might be transported more efficiently or that the protein might work more efficiently in some yeast strains.
But none of these theories explained why the synergistic results could not be replicated, McLachlan said, adding, "I don’t know why."
McLachlan said he felt it important to publish a formal retraction, because "a lot of people have given this study a lot of accord in the public policy debate."
Gibbons says ‘scientific process moves forward’
John Gibbons, director of the White House Office of Science and Technology Policy, referred to McLachlan’s retraction at the July 21 session of Estrogens in the Environment IV, a conference hosted by NIEHS. There are many uncertainties surrounding endocrine disruptors, Gibbons said. As research continues, "some observations will be found true and others found not valid." Tulane has been unable to replicate its results, Gibbons continued, and "the scientific process moves forward."
McLachlan is best known for his research on diethylstilbestrol, a drug used in the 1950s that caused vaginal cancer in the daughters and reproductive abnormalities in the sons of women who had taken the drug during pregnancy. The discovery that DES could harm offspring who were exposed in the womb foreshadowed current interest in endocrine disruptors.
REF: Food Chemical News, 39(23):9-10, July 28, 1997.
Scientists Challenge Aspartame/Brain Tumor Link in Children
A group of scientists, writing in the July 16 issue of the Journal of the National Cancer Institute, reported that they found no evidence "to support the hypothesis that consumption of aspartame is related to pediatric brain tumor incidence."
As part of a population-based case-control study of environmental and nutritional risk factors for pediatric brain tumor occurrence, the scientists collected data on aspartame consumption before the date of diagnosis for case patients, or a comparable reference date for control subjects.
The analysis was conducted on 56 case patients (children who developed brain tumors) and 94 control subjects who were born in 1981 or later (after aspartame was approved for general use in the USA). In addition, they also evaluated brain tumor risk relative to the mother’s consumption of aspar-tame and breast-feeding.
"Case children were no more likely than control children to consume foods containing aspartame, either from all sources of aspartame combined or from diet drinks," Gurney et al. said. Furthermore, there was "no suggestion of a dose-response relation" based on age at first consumption, number of years of consumption, or frequency of consumption. Maternal consumption of aspartame during pregnancy also appeared to have no effect on brain tumor risk to the child.
While acknowledging that their study sample was small and confidence intervals of risk estimates relatively wide, the authors said that their findings were "not consistent with an aspartame-brain cancer relation."
The authors said that when evaluating exogenous agents that require a long interval between exposure and carcinogenic effect, studies in children have limitations. As such, they said they could "not rule out the possibility that children in our study who were exposed to aspartame will incur an increased brain tumor risk as adults." But, they said, given the almost simultaneous occurrence of the peak rise in brain tumor incidence rates with the introduction of aspartame into public foodstuffs, which is inconsistent with the usual latency periods of solid tumor carcinogens, "it appears unlikely that any carcinogenic effect of aspartame ingestion could have accounted for the recent brain tumor trends as Olney et al. contend."
REF: Food Chemical News, 39(23):10-11, July 28, 1997.
Airbags: Benefits and Risks
This article from the July 1997 issue of Risk in Perspective, summarizes what has been learned about the benefits and risks of airbags from real-world crash experience in the U.S. They compare the airbag’s actuarial record (i.e., the historical counts of deployment incidents and their outcomes) with what it was projected to be by analysts 10-20 years ago. They also discuss how airbag technology might change in the future in order to maximize safety benefits while reducing the unwanted risks to motorists.
Lifesaving Benefits
In 1977 the U.S. National Highway Traffic Safety Administration (NHTSA), predicted, based on experimental testing, that airbags would reduce the overall risk of fatal injury by 40% among unbelted occupants and by 10% among belted occupants. Drivers and front-right passengers were predicted to reap similar benefits and no distinctions were made about occupant characteristics (i.e., age, sex or height).
Based on actuarial data accumulated in the USA since 1986, it appears that the qualitative predictions were correct: airbags are saving lives. Death rates among drivers are lower in cars with airbag systems and these differences are not explainable by other vehicle or behavioral variables. However, the magnitude of the airbags’ lifesaving effect for the unbelted driver is much smaller than projected (about 13% instead of 40%).
Through post-crash investigations it has been learned that airbags have caused the deaths of at least 19 drivers who were involved in crashes of low-to-moderate severity. These drivers were predominantly short women over the age of 60 and it should be empha-sized that most of them were not wearing safety belts when the crash occurred.
Overall, the best available estimates are that the lives of 75 drivers are being saved for each one that is being lost due to airbags.
Airbags are also saving the lives of adult front-right passengers. The net effectiveness estimates reported for front-right occupants who are older than 13 years old are similar to the actuarial estimates reported for drivers.
There is, however, one subgroup of passengers that is experiencing a net increase in fatality risk due to the introduction of airbag technology. Post-crash investigators have reported that at least 40 children under the age of 10 have been killed by passenger-side airbags in crashes that would not typically have been fatal. Most of these children were either unrestrained or improperly restrained. Although the lives of some children may have been saved by airbags, the best actuarial estimates are that the presence of a passenger-side airbag in a vehicle is associated with a net increase in the risk of child mortality of 21% to 88%.
Overall, it appears that about 40% of airbag deployments result in at least one occupant injury (although most of these injuries are minor).
Public Perceptions and Attitudes
In a March 1997 telephone survey of 1,000 licensed drivers, the Harvard Center for Risk Analysis found that a majority of USA residents maintain favorable attitudes toward airbags, despite a significant amount of unfavorable media coverage in the year prior to the survey. A substantial majority (64%) favor the current legal requirement that all new vehicles be equipped with dual airbags.
Although most citizens perceive correctly that airbags save more lives than they kill, a majority (60%) harbor the misconception that airbags save more lives of children than they kill. Most respondents (74%) are also unaware that a properly belted occupant (especially one who sits very close to the steering wheel) can suffer moderate to serious injury in a low-speed crash from an inflating airbag. And people do not realize that airbags are designed to deploy even in relatively low-speed crashes where the need for airbag protection is questionable, particularly if people are wearing safety belts.
REF: Risk in Perspective (Harvard Center for Risk Analysis), 5(7), July 1997.
Microwave Oven Found Effective in Disinfection of Kitchen Sponges and Dishcloths
Microwave heating was reported to be a very efficient method for decontaminating cellulose sponges and cotton dishcloths that are believed to harbor bacteria and cross-contaminate other food contact surfaces in the kitchen.
P.K. Park, of the University of Wisconsin’s Food Research Institute in Madison, Wis., and D.O. Cliver, of the Department of Population Health and Reproduction at the University of California in Davis, Calif, reported in the March 1997 issue of Dairy, Food and Environmental Sanitation that exposures of 60 seconds in a common, household microwave oven were sufficient to kill 107 bacteria.
Hypothesizing that the high temperature induced by the microwave energy may be responsible for the high kill, the researchers said that microwave heating is a simple and potent way of disinfecting sponges and dishcloths -- important food-contact surfaces in the home kitchen.
Bacteriological survey of used sponges and dishcloths reported
In the January 1997 issue of the same journal, Carlos Enriquez and colleagues at the University of Arizona in Tucson, Ariz., reported the findings of a bacteriological survey of 325 sponges and 75 cotton dishcloths collected from households in four major U.S. cities.
A total of 23 different bacterial species were identified from 140 cellulose sponges and 13 from 56 dishcloths. Most identified species belonged to the Enterobacteriaceae, Pseudomonas spp and Burkholderia spp groups. No definite identification could be obtained from 82 isolated colonies.
Salmonella spp was identified in 15.4% (13 of 84) and 13.8% (4 of 29) of the cellulose sponges and dishcloths, respectively. Staphylococcus aureus was present in 20% (65 of 325) of the cellulose sponges, and in 18.6% (14 of 75) of the dishcloths, according to the researchers, who stated that cellulose sponges and dishcloths may be an important source of bacterial contamination of surfaces, hands, and foods in home kitchens.
REF: Food Chemical News, 39(18):5, June 23, 1997.
Effects of Processing on Pesticides, Heavy Metals, PCBs, Animal Drugs, Mycotoxins in Foods Discussed at ACS Session
While processing can virtually eliminate pesticide residues on fruits and vegetables, other food contaminants, such as heavy metals, PCBs, animal drug residues and mycotoxins, are more difficult to destroy, researchers said during a session of the American Chemical Society’s (ACS) conference in San Francisco in April, 1997.
"Residues are rarely detected on processed fruits and vegetables," said Henry Chin of the National Food Processors Association in Dublin, Calif.
Nonetheless, experts from around the country discussed the effects of processing on various food contaminants, and found their complete removal by traditional processing methods to be a difficult task.
A large portion of raw fruits and vegetables in the United States are processed, Chin said: About 85% of tomatoes, for salsa and sauces; 42% to 45% of apples, for juice and sauce; and 76% to 79% of oranges, for juice.
To begin with, Chin said that "quite often, the residues on fresh fruits and vegetables are postharvest pesticides" to keep produce pest-free while it is transported to the market. However, when fruits and vegetables are processed, there is no need for post-harvest applications because the time between harvesting and processing is so short.
In his presentation, Chin argued that techniques such as washing, sorting, peeling, blanching and canning "generally can dramatically reduce the amount of residues" remaining on processed fruits and vegetables.
In one commercial study, residues from pesticides such as methyl parathion and chlorpyrifos were found on 10 samples of raw unwashed apples. After being processed into apple juice and sauce, "no residues were detected in any of the finished samples," Chin said.
Processing usually reduces PCBs in fish
Likewise, processing and cooking usually reduces the levels of polychlorinated biphenyls (PCBs), polybrominated biphenyls (PBBs) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in fish and fish oils, said Mary Zabik of the College of Human Ecology at Michigan State University.
PCBs accumulate in waterway sediments, and are redistributed by dredging, boating, storms and the fish themselves, Zabik said. "These are lipid-soluble compounds, so processes that reduce fat are also effective in reducing the levels of environmental contaminants. "
In one study of PCBs in fish caught in the Great Lakes, Zabik found that baking, broiling and pan-frying reduced PCB levels 20% to 35%. For TCDD, smoking reduced the levels 100% in carp, while baking, char-broiling and pan-frying cut down the contaminant about 45%.
Containers, cooking cause lead leaching in food
For other contaminants, processing often increases rather than decreases levels. For example, Jeffrey Morgan of EPA’s National Exposure Research Laboratory in Cincinnati said the toxic heavy metals content of foods depends on factors ranging from the metal content of soils to packaging materials and preparation techniques.
Morgan presented the results of numerous tests showing how toxic metals get into food and stay there. For example, grapefruit juice stored in tin-coated steel containers for two weeks had higher accumulations of lead. "If you’re going to store grapefruit juice, it should be in some other kind of container, either glass or plastic."
With coffee prepared in ceramic mugs, half of the lead leached during the first half hour, with lower levels of leaching for up to 24 hours. Additionally, old dinnerware with lead-containing glazes will leach high levels of lead into food in the microwave. "These types of dinnerware should not be used for heating in the microwave. They weren’t designed for that."
Morgan’s lab tested the effect of various cooking techniques on the mercury content of walleye fillets. Whether raw, pan-fried or smoked, "the mercury isn’t going anywhere. It remains through the cooking process."
Arsenic, which is commonly used as a growth enhancer for chickens, can accumulate in egg yolks and albumen. As with mercury, cooking does not lower arsenic levels and in some cases concentrates the toxic metal.
Veterinary drug residues in cooked meats vary
While veterinary drug residues are usually identified in raw meats, most animal foods are cooked or processed before they are eaten, said William Moats of the USDA/ARS Meat Science Research Laboratory in Beltsville, Md. In a review of numerous studies, Moats found that the degradation of residues varies widely depending on the type of compound and the cooking method.
"In ordinary cooking of meat by procedures such as frying, grilling, or roasting even to ‘well-done,’ temperatures in the center did not exceed 80oC-90oC. These processes resulted in some degradation of compounds such as oxytetracycline, chlortetracycline and penicillin G, depending on time and temperature of heating. Sulfamethazine and clenbuterol were stable under these conditions."
To completely degrade veterinary drugs, most contaminated meats needed to be cooked for up to several hours at 100oC in buffers, meat extracts, milk or water. In addition, residues are rarely distributed evenly in meat tissues, causing additional variability.
"There’s very little reduction when heating to rare, and more significant reductions when heating to well-done. The results vary from medicine to medicine and with cooking methods and times."
Some mycotoxins easier to destroy than others
L.B. Bullerman of the Department of Food Science at the University of Nebraska also found considerable differences in the effectiveness of processing methods on the destruction of mycotoxins on grains.
Mycotoxins are low molecular weight compounds produced by molds; some are acutely or chronically toxic while others are considered carcinogens. In grains, they include fumonisins, deoxynivalenol, aflatoxin and zearalenone. Some 400 mycotoxins are currently known.
"There is a considerable amount of research devoted to trying to save mycotoxin-contaminated crops. Methods for reducing toxins include breeding, controlling storage conditions and physical, chemical or biological decontamination. Different processing methods yield a variety of results. With aflatoxin, for example, hand sorting can work, with low levels of contamination remaining; thermal processing produces at best, only small reductions; roasting yields a small reduction; and ammoniation can be somewhat effective but is not approved by the FDA for human food."
"A number of safe and effective methods exist for reducing mycotoxins in food. However, none of them completely removes all of them," Bullerman concluded.
REF: Food Chemical News, 39(13):9-11, May 19, 1997.
Total Pesticide Ban Ordinance Enacted
By the year 2000 the city of San Francisco will ban the use of all pesticides on city property -- outdoor parks, golf courses, public housing and any building the city owns. Private property and buildings are exempt from this ordinance. In brief:
Phase I: The first part of the ordinance prohibits the use of all Toxicity Category I pesticides -- those proven to be highly toxic -- as well as pesticides believed to be possible human carcinogens or the cause of reproductive disorders as of January 1, 1997.
Phase II: The second part of the phase-out requires the city to reduce remaining pesticide use by 50% by January 1, 1998. The Board of Supervisors ruling also requires a 4-day notification of pesticide applications before and after spraying and improved recordkeeping of pesticide use by city departments.
During the transition to pesticide elimination, the city must implement an Integrated Pest Management (IPM) program. This type of program would classify the use of pesticides as a "last resort" when everything else has failed.
Neither the grounds-care industry or sound science supports this kind of program. In science-based IPM programs, you consider all the appropriate methods of control and select the ones that will provide the best control. Many may wonder, then, why go to the effort of initiating an IPM program to reduce pesticides when the ultimate plan is to ban them altogether?
Phase III: At the commencement of the new millennium, the only pesticides permitted for use in San Francisco will be those the Board of Supervisors has reviewed and approved for use for purposes of public safety and public health.
San Francisco is already experiencing a lot of pressure to make exceptions to the new pesticide guidelines. Grounds managers and other city workers have already made their way to the commissioners to educate them on the harm that their decision will have on the city of San Francisco.
REF: Kansas Pesticide Newsletter, 20(6):33, June 10, 1997.
DPR Report: Amount of Pesticides Sold in 1995
There were 543.1 million pounds of pesticide* active ingredients reported sold in California in 1995, according to a summary released by Cal/EPA's Department of Pesticide Regulation (DPR).
Products that control microorganisms or algae in water -- including sanitizers, disinfectants, and bactericides -- make up the majority of pesticides sold. The largest amounts of such chemicals are chlorine-based products used in swimming pools, lakes and ponds, and in water used for manufacturing processes. Some 53% of the pesticides sold in 1995 were these algicides and antimicrobials.
Fungicides accounted for the second largest category of pounds sold at 21%. Such products control mildews, molds, and pathogens that may cause diseases in plants, animals, and humans. Fungi also may destroy wood and fiber products if left untreated.
(*Pesticide is an umbrella term used to describe many kinds of chemicals that control or repel pests, including insecticides, fumigants, nematicides, rodenticides, defoliants, growth regulators, herbicides, bactericides, antimicrobials, algicides, and fungicides. Adjuvants --substances like emulsifiers added to pesticides to enhance their effectiveness-- are also considered pesticides under California law.)
REF: Department of Pesticide Regulation Release No. 97-24, September 3, 1997.
Methyl Bromide, "Air Time"
The National Oceanic and Atmospheric Administration (NOAA) has announced findings that methyl bromide remains in the atmosphere for LESS time than scientists previously thought. This conclusion stems from the finding that oceans remove more methyl bromide from the air than was believed.
NOAA researchers found that the atmospheric lifetime of methyl bromide (how long the chemical stays in the atmosphere before it breaks down or is removed) is about 8.5 months. Up until now, it was believed that methyl bromide’s atmospheric lifetime was more than 24.0 months. The shorter atmospheric lifetime was calculated after researchers discovered that the ocean absorbs methyl bromide from the atmosphere at a rate faster than previously thought.
Methyl bromide can be removed from the atmosphere by two major, natural processes. It is chemically converted in the atmosphere through oxidation, and it is absorbed by the ocean. The loss of atmospheric methyl bromide to the ocean is just about as fast as oxidation in the atmosphere.
The U.S. is the world’s biggest user of methyl bromide. Plans are to phase out the chemical here in the year 2001. By contrast, the European Union, the second biggest user, only plans to cut consumption by 25 percent by 1998 and phase it out by 2015. The third largest user, Japan, has no plans at all to reduce or phase out methyl bromide.
REF: Kansas Pesticide Newsletter, 20(8), August 20, 1997.
Air Standards Not to Affect Farming
EPA Administrator Carol Browner sought to assure the Senate Agriculture Committee that farmers will not be targeted in enforcement of tougher air quality standards for ozone and particulate matter promulgated by the agency July 16, 1997.
"Fears and concerns we’ve heard about the effects on agriculture have been based on misconceptions and misinformation," Browner told the committee at a July 22 hearing. "EPA is not going to regulate farmers to reduce fine particulates in the air or to control ozone. We will not restrict tilling. We will not regulate ammonia emissions from animal wastes. And there isn’t going to be any wholesale action against agricultural burning on private lands," Browner declared. States won’t focus on farmers, either, Browner added, "It wouldn’t make sense to target agriculture. Tilling practices are such a tiny, tiny part of this."
But witnesses representing grower organizations as well as some committee members were not persuaded by Browner’s testimony.
"Don’t let anybody tell you agriculture is not going to be affected by these standards," said Phillip Wakelyn, manager of environmental health and safety for the National Cotton Council.
Senator Pat Roberts (R-Kan.) said he was concerned about the possible impact on tractor emissions and pesticide and fertilizer applications when the standards are implemented. Sen. Charles Grassley (R-Iowa) questioned the need for the standards, asserting that "some very respected members of the scientific community" are skeptical about the new regulatory initiative.
Committee Chairman Dick Lugar (R-Ind.) said at the outset of the hearing that he shares the concern of the agricultural community that "the science behind the regulations is inadequate."
Browner, however, described the new standards as "the most significant step we’ve taken in a generation to protect the American people — and especially our children — from the health hazards of air pollution." "Clearly," she testified, "the best available science shows that the previous standards were not adequately protecting Americans from the hazards of breathing polluted air. Revising the standards will bring enormous health benefits to the nation."
In her prepared statement, Browner pointed out that implementation of the new standards is years away. EPA must develop and install a national air monitoring network — a three-year project in itself — then accumulate data for another three years before designating any nonattainment areas. If such designations are made, states will have another three years to develop control programs, she said.
REF: Kansas Pesticide Newsletter, 20(8), August 20, 1997.
"TOX TIDBITS"
Knowledge and Use of Folic Acid by Women of Childbearing Age — United States, 1997
Each year in the U.S., approximately 4000 pregnancies are affected by spina bifida and anencephaly. Babies born with spina bifida usually survive, often with serious disability, but anencephaly is invariably fatal. The B vitamin folic acid can reduce the occurrence of spina bifida and anencephaly by at least 50% when consumed daily before conception and during early pregnancy. Folic acid can be obtained from multi-vitamins or certain other supplements and from some fortified breakfast cereals. It is found naturally in orange juice, green leafy vegetables, and beans; however, it is difficult to obtain the recommended 400 mg daily through diet alone.
Editorial Note: The 1995 Gallup Organization-March of Dimes survey found a relatively low awareness of folic acid and of the Public Health Service recommendation, illustrating the need for educational strategies to inform more women about the benefits of folic acid.
REF: Morbidity and Mortality Weekly Report, 46(31):721-723, August 8, 1997.
Umbrella Recall
Gymboree Corporation is recalling about 6,500 children’s compact umbrellas and about 700 beach and golf umbrellas because they pose a lead-poisoning danger to young children due to decorations that contain high levels of lead. The umbrellas have patterned panels that alternate with solid-color panels; the children’s model has a clown on the purple panel. The umbrellas were sold for $12 to $30 from September 1996 to May 1997. For more information, consumers can call Gymboree at 800-558-9885.
REF: Washington Post, August 13, 1997.
Phone Risks
An article in the New England Journal of Medicine examined the dangers of using car phones. A related article in the February 1997 issue of the journal discussed how various scanning technologies helped medical researchers at the Hospital Broussais in Paris identify pooling blood that was blocking a woman’s carotid artery. The cause -- extended use of a portable phone while doing chores.
The 39-year-old healthy woman experienced problems shortly after having a 32-minute telephone conversation while ironing. She held the phone to her head by raising her shoulder. Shortly after hanging up, she felt sudden severe neck pain, followed by ringing in her ears. The pain lasted 20 hours, the ringing, 48 hours. At the hospital, CAT scans, Doppler ultrasound, and magnetic resonance (MR) angiography revealed pooling blood in the artery causing "extracranial right internal carotid artery dissection." Digital intravenous angiography showed that the woman did not have underlying artery disease. MR angiography performed several weeks after the pain ceased showed that the state of the carotid artery had returned to normal.
According to the researchers, internal carotid artery dissection is a major cause of stroke in young people. It is usually caused by sports injuries, hypertension or the head being yanked in an unnatural direction. They warn that extensive use of cordless phones while doing other things can produce head movements that can damage the carotid artery. (New Eng. J. Med., Feb 13, 1997, p. 516.)
REF: Florida Veterinary Scene Veterinary Medicine Newsletter, 6(7):7, July 1997.
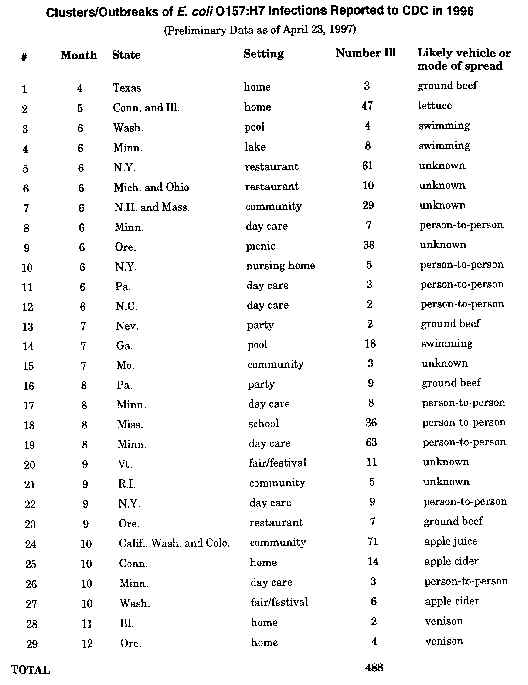
Outbreaks of Escherichia coli O157:H7 Infection Associated with Eating Alfalfa Sprouts
In June and July 1997, simultaneous outbreaks of Escherichia coli (E. coli) O157:H7 infection in Michigan and Virginia were independently associated with eating alfalfa sprouts grown from the same seed lot. The outbreak strains in Michigan and Virginia were indistinguishable by molecular sub-typing methods.
Editorial Note: These are the first reported outbreaks of E. coli O157:H7 infection associated with eating alfalfa sprouts. Since 1995, four outbreaks of Salmonella infection have occurred in the U.S. because of consumption of contaminated alfalfa sprouts. In 1996 in Japan, radish sprouts were associated with the largest recorded outbreak of E. coli O157:H7 infection, in which approximately 6000 cases occurred.
The recurrent implication of alfalfa sprouts as a vehicle for foodborne illness highlights the need for strengthened prevention and control measures to ensure the safety of this product. Studies of alfalfa seed inoculated with low numbers of Salmonella suggest that the number of organisms present on seeds may increase up to 10,000-fold during the sprouting process. The effect of the sprouting process on the growth of E. coli O157:H7 is unknown.
REF: Morbidity and Mortality Weekly Report, 46(32):741-743, August 15, 1997.
E. Coli Survival, Detection Subject of Posters at American Society of Microbiologists Meeting
Disease-causing E. coli bacteria have a better chance of surviving in frozen hamburgers than in refrigerated patties.
Storing ground beef at temperatures between 28oF and 36oF, instead of -4oF, may help reduce the spread of pathogens in food. E. coli O157:H7 has a better chance of survival in subzero temperatures than once thought. The greatest reduction of E. coli populations occurred when the meat was held at 59oF for four hours, then reduced to 28oF for five weeks.
REF: Food Chemical News, 39(15):5-7, June 2, 1997.
E. coli O157:H7
Illness caused by E. coli O157:H7 infection usually is characterized by abdominal cramping, diarrhea, and bloody stools and can be complicated by hemolytic uremic syndrome and death. Persons with illness meeting this description (i.e., abdominal cramping, diarrhea, and bloody stools) should contact their physicians.
REF: Morbidity and Mortality Weekly Report, 46(33):777-778, August 22, 1997.

REF: Food Chemical News, 39(13):17, May 19, 1997.
Reye Syndrome and Aspirin
Reye syndrome is rare and associated frequently with certain viruses. Thus, comparison of medication histories in cases and controls who had the same viral infection may be important. In two studies, controls were selected from the same school and had a prodromal illness within 1 week of that of the cases. It is probable that many cases and controls were matched for infection because a large percentage of the cases occurred during outbreaks of influenza, and varicella patients were matched with other children who had varicella. Further analysis of the salicylate association by specific type of infection should be possible in one of the studies.
In 1976 the Food and Drug Administration advised that, when treating children who develop vomiting associated with a viral illness, caution should be exercised in using acetaminophen, salicylates, and antiemetics because of the suspicion that these drugs, in combination with a viral illness (a possible cause of vomiting in children) might contribute to the development of Reye syndrome. The results of these studies suggest that during certain viral illnesses the use of salicylates — even before the onset of vomiting — may be a factor in the pathogenesis of Reye syndrome. In view of these data, parents should be advised to use caution when administering salicylates to treat children with viral illnesses, particularly chickenpox and influenza-like illnesses.
REF: Morbidity and Mortality Weekly Report, 46(32):750-753, August 15, 1997.
A Mosquito Bites Back
According to the World Health Organization, mosquitoes will kill 1 out of every 17 people currently alive. Mosquitoes transmit disease to 700 million people every year, and cause about 3 million deaths. A mosquito that was once thought to have been eradicated from the Western Hemisphere, Aedes aegypti, has reappeared and the consequences may be severe. The threat of urban yellow fever is being taken seriously by the Centers for Disease Control and the National Institutes of Health.
REF: New York Times Magazine, August 24, 1997.
Smog on the Job: Humans, Perfumes, Chemicals, and Electronic Equipment Don't Always Live Happily Together
Alun M. Anderson, editor of New Scientist, writes about "office smog," clarifying how volatile organic compounds (VOCs) from perfumes, glues, dry-cleaned clothes, etc. combine with ozone produced by electronic equipment (faxes, photocopiers, printers) to create hydroxyl radicals which "are wildly destructive and tear apart other VOCs to create still more harmful chemicals...These chemicals attack human and machine alike." He notes some upcoming reports that will address this issue: a European Union checklist of 60 airborne chemicals suspected of being injurious to human health and EPA's Building Assessment Survey and Evaluation (BASE). He concludes by calling on EPA and OSHA "to accelerate their efforts to set standards for office air quality, and to provide guidance about how they can be achieved."
REF: Washington Post (Commentary), August 28, 1997.
Benefits of Pesticide Use on Strawberries and Sex
According to a news brief, a woman in Winnipeg, Canada, who nearly swallowed a worm in her strawberry yogurt has now been suffering from a depressed sex drive as a result and is suing a grocery chain for negligence. The woman alleges that the grocery store chain that sold the strawberry yogurt was negligent in not ensuring that the yogurt was worm free or at least had warning labels.
REF: Kansas Pesticide Newsletter, 20(9), September 15, 1997.
Another Example of the Dose-Response Relationship
Physicians James J. Chamberlain and Igor Z. Abolnik report in the September issue of the Western Journal of Medicine that binge eating of licorice can cause pulmonary edema, or swelling caused by excess fluid around the lungs. Licorice contains glycorrhizic acid, which is known to cause heart problems, including congestive heart failure. The man in the case study ate four packages (about 2 ¼ pounds) of Twizzlers® over a period of three days.
REF: Washington Post Health, September 30, 1997.
![]() Vet Notes
Vet Notes
![]()
The Effect of Feeding Dried Tomato Vines to Beef Cattle
Abstract
Dried tomato vines (DTV) are used as a feedstuff in some herds of beef cattle in Israel, despite the literature citations that tomato vines contain potentially harmful steroid alkaloids. A small-scale feeding trial over 42 days examined possible deleterious effects of feeding DTV, compared with wheat straw, in beef cows. No differences in hematological values, serum parameters or body weight were seen between the 2 groups. Steroid alkaloid content of the DTV was not examined, but toxic levels of nitrates were found. The main practical hazard in feeding DTV would appear to be from their potentially high nitrate content.
In arid areas of the eastern Mediterranean basin, where no rain falls from May to October, natural pastures for grazing beef cattle are insufficient even for maintenance, and supplementation of their ration has to be implemented. As the profit margin of this effort is low in Israel, beef farmers are increasingly looking for low-cost, often unconventional feedstuffs. The most common of these is dried poultry litter (DPL), which although it is an excellent supplement nutritionally has occasionally been problematic from a veterinary health viewpoint and has caused cattle botulism, maduramicin toxicosis and copper poisoning. Another unconventional feedstuff, dried tomato vines (DTV), has been used in Israel on a small scale to supplement the diet of beef cattle towards the end of the long dry summer when pasture is scarce. As some 2400 tons of DTV/year are available in Israel, it is worthwhile to examine this product from nutritional and toxicological aspects to demonstrate that it is not deleterious to cattle health and can be safely used as a feedstuff. This small-scale feeding trial examined this question.
Materials and Methods
Ten non-pregnant beef cows of mixed parentage were randomly assigned to 2 groups, a control group and the dried tomato vines (DTV)-fed group, each comprising 5 cows. The groups were fed a maintenance ration comprising barley and DPL, with the addition of wheat straw given ad libitum to the control cows and DTV supplied ad libitum to the DTV group. The cows were acclimatized to their new ration over several days. The DTV was from a normal commercial crop of tomatoes.
The amount of wheat hay and DTV consumed by each cow was measured daily. The feedstuffs were examined for nutrients and nitrates; a pesticide screen was conducted by extraction with hexane and examination by gas chromatography with a nitrogen/phosphorus specific detector. The cattle were weighed several times before and during the trial, and blood samples were taken from all the cows before the feeding trial and twice thereafter, at 14 and 40 days. The trial continued for 42 days. Blood was examined for hematological parameters (red blood cell count, hematocrit, hemoglobin, white blood cell count), and for the serum parameters of albumen, total protein, alkaline phosphatase, aspartate aminotransferase, creatine phosphokinase, gamma-glutamyl transferase, lactic dehydrogenase, calcium, magnesium, sodium, potassium, chloride, phosphorus, cholesterol, creatine and urea, as well as progesterone. Statistical analysis of parameters was conducted by the Student’s t-test and ANOVA.
RESULTS
Feedstuffs
The wheat straw had 11% moisture, 3.5% protein, 7.1% ash, 40.6% crude fiber and trace levels of nitrates (<0.05%). The DTV contained 11.5% moisture, 8.4% protein, 17.9% ash, and 31.2% crude fiber; nitrates were found in the fruit (<0.05%), the thick stems (0.1%), the thin stems (0.7%) and the leaves (1.1%). Thick and thin stems comprised most of the DTV on a weight basis. The pesticide screen, which particularly detects organophosphorus and carbamate insecticides, did not reveal any N- or P- containing pesticides.
Cattle Health
Feed eaten (/head/day) averaged 3 kg DPL, 3 kg barley, and 5 kg wheat straw or 5.5 kg DTV. The control group lost an average weight of 0.68 kg/head/day throughout the trial, whereas the DTV group lost an average of 0.60 kg/head/day over the same period; these differences were not significant (P<0. 05). No signs of ill health were seen in any of the cows, but animals in both groups were regarded as being visibly underweight by the end of the trial.
Blood Parameters
No consistent changes in any of the blood parameters were found between the wheat straw and DTV groups over the period of the trial. Some changes (P<0.05) from pre-trial blood parameters were found in both groups; these were elevated urea levels and lowered sodium, potassium and chloride blood levels.
Discussion
The tomato, Lycopersicon esculentum (Solanum lycopersicum), as one of the Solanaceae family is considered to be potentially toxic. Many of the Solanaceae contain steroid alkaloids, particularly solanine, which is hydrolyzed to the aglycone solanidine and toxic saponins. Tomato plants contain the glycoside tomatine, which is also toxic. Concentrations of toxins in plants are highly variable and totally unpredictable with variations in sub-species (strains of plant or vegetable), soil type, season, water supply and nutrients available capable of causing up to 100-fold differences in toxin levels; the toxic constituents of tomato plants probably vary under similar circumstances. The literature contains very little on the toxicity of tomato vines to cattle, with only dated anecdotal references to field outbreaks. In both cases mortality was caused consequent to eating unripe fruit and vines, in 1 case in 8 calves and in the other case in 8 calves and 2 cows; no details were given of clinical signs, necropsy findings, or of a differential diagnosis.
Poisoning consequent to feeding DTV has been diagnosed in Israel when cattle showed clinical signs (with mortality and abortion) and necropsy and laboratory findings indicative of nitrate poisoning. In those cases, high levels of nitrates (more than 2% on a dry weight basis) were found in the DTV. Nitrates are also suspected of having a chronic effect on cattle fertility by depressing blood progesterone levels, as well as other possible but largely unproven deleterious effects.
The results of this trial did not indicate that DTV have any deleterious effect on cattle health when fed ad libitum. The hematological and biochemical tests did not suggest that any damage was caused to any organ systems. Blood progesterone levels depend upon at what stage of the estrus cycle the blood sample is taken, but this was not examined in the cows in this trial; it therefore could not be conclusively stated that the nitrates in the DTV did not affect progesterone levels. Tomatine may inhibit cho-linesterase, but this was also not examined. There were reductions in body weights in both groups in the trial. This may be related to the fact that prior to the trial the cows had been fed DPL which was not examined for, and therefore may have contained, deleterious levels of ionophores.
An overall assessment of the potential toxicity of DTV is not easy. The GC screen eliminated possible contamination with many potentially harmful pesticides which might have been sprayed on the plants during their growing period. No effort was made to determine levels of steroid alkaloids, aglycones, saponins or other toxins; such data would have been relevant only for that particular crop of tomato plants, for reasons explained above. Also the complexity of such analyses precluded such testing on a routine basis. However, the absence of any changes in blood parameters indicated that even ad libitum feeding with this DTV was not evidently deleterious.
Of more concern was the level of nitrates found in DTV in Israel, in this trial and in other samples. The lowest levels of nitrates were found in the thicker stems, which made up a large part of the fiber content in DTV; however, the cows often rejected these parts, preferring the thin stems and leaves which contained higher levels of nitrate. Nitrate levels of 0.5% may be associated with chronic effects such as infertility; levels of 1% have caused abortion in acute toxicoses; and levels of 1.5% have caused fatal peracute poisonings. Growing tomatoes on high nitrate soils, or with nitrogen-based fertilizers, will likely induce elevated plant nitrate levels, and then DTV might be hazardous to cattle, particularly if fed without prior acclimatization to the nitrates in DTV. (Shlosberg, et. al.)
REF: Veterinary and Human Toxicology, 38(2):135-136, April 1996.
FDA Bans Extra-label Animal Drug Use of Fluoroquinolones and Glycopeptides
May 22, 1997, FDA issued a final rule/order of prohibition forbidding the extra-label use of fluoroquinolones and glycopeptides because the agency believes that some extra-label uses of these in food-producing animals are capable of increasing the level of drug-resistant zoonotic pathogens in treated animals at time of slaughter.
FDA also found that some extra-label uses in food-producing animals will likely cause an adverse event, resulting in a finding of a risk to public health under the Animal Medicinal Drug Use Clarification Act of 1994.
In issuing the final rule, FDA said the fluoroquinolones, sarafloxacin and enrofloxacin, were approved for therapeutic use in poultry. These approvals for use in food animals were conditional on use under a veterinarian’s supervision to reduce the rate of emergence of sarafloxacin-resistant organisms. FDA added that its Veterinary Medicine Advisory Committee and the Center for Drug Evaluation and Research’s Anti-Infective Drugs Advisory Committee supported restrictions of the drugs in order to maximize benefits and minimize risks related to resistant organisms.
FDA noted that one glycopeptide, vancomycin, is approved for use in human medicine and none are approved for animal use. As a practical matter, therefore, the agency’s prohibition against extra-label use in animals of glycopeptides applies only to vancomycin. The prohibition will, however, apply to any future animal drug approvals of glycopeptides, FDA added, unless the circumstances at the time of approval cause a reevaluation of any part of the prohibition.
The order of prohibition became effective August 20, 1997.
REF: Food Chemical News, 39(14):9-10, May 26, 1997.
Antibiotic Milk Isn’t Reaching Store Shelves
During FDA’s three fiscal years, 1994 through 1996, 9,735,154 bulk milk pickup tankers were screened for beta-lactam (penicillin is a beta-lactam) drug residues. Only 8,789 were positive. The good news is that 99.91 percent passed the screening test. The bad news is that 0.09 percent did not.
The Pasteurized Milk Ordinance (PMO), the document which is developed jointly by the industry and FDA, is adopted by states and becomes, with minor revisions, the Grade A milk law in all states. The PMO requires that all bulk milk pickup tankers be sampled and analyzed for beta-lactam animal drug residues before the milk is processed. In addition, other drugs are screened voluntarily, and those results are included in the data also.
The really great news is that antibiotics are not getting into finished products on store shelves. During the same time period, 78,000 finished-product tests were conducted. Only two were positive; they came from a non-Grade A jugger.
The most farm samples are tested during the second quarter of the year, April through June, high milk production months. January through March and July and September are the highest percent positive months. During the winter, treatments and resulting residues probably are due to respiratory infections. Late summer positives most likely are due to mastitis treatments.
Joseph Smucker, chief, Milk Safety Branch, FDA, offered the above information at the annual meeting of the National Mastitis Council. Near the end of his presentation, he concluded, "The system works."
That’s the point. The system works. Drug residues are not a perceived or real threat in consumer packages of milk and other dairy products. All the industry can be thankful the headlines are over.
REF: The University of Georgia Veterinary Newsletter, No. 337, August 1997.
"VET TIDBITS"
Zoonotic Transmission of Ascarids and Hookworms
Ascarids (Toxocara spp.) and hookworms (Ancylostoma spp. and Uncinaria stenocephala), the common intestinal roundworms of dogs and cats, can cause larva migrans syndromes in persons who accidentally ingest eggs or larvae or have direct skin contact with hookworm larvae in soil contaminated with the feces of infected animals.
The common ascarid of dogs, T. canis, has long been recognized as a cause of larva migrans syndromes in children. The ascarid of cats, T. cati, can also cause disease in humans, although for reasons partly related to the "toilet behavior" of cats, it does so less frequently than T. canis. When the eggs are accidentally ingested, they hatch, and infective-stage larvae migrate through human liver, lungs, and other organs and tissues where they produce damage and induce allergic responses. Infection may leave children with permanent visual or neurologic damage.
In the U.S., the popularity of pets together with high ascarid and hookworm infection rates in dogs and cats, especially pups and kittens, result in widespread contamination of soil with infective-stage larvae. Epidemiologic studies have implicated the presence of dogs, particularly pups, in the household and pica (dirt eating) as the principal risk factors for human toxocaral disease. Children’s play habits and attraction to pets puts them at high risk for ascarid and hookworm infection.
Hookworms of dogs and cats, A. caninum, A. Brazilense, A. tubaeforme and U. stenocephala, can also infect people when larvae in soil are ingested or directly penetrate the skin on contact. Cutaneous larva migrans syndromes, characterized by progressive, intensely pruritic, linear eruptive lesions caused by prolonged migration of the larvae in the skin, are the most common manifestations of zoonotic hookworm infection. A. caninum larvae may penetrate into deeper tissues, however, and induce symptoms of visceral larva migrans or migrate to and partially mature in the intestine, inducing eosinophilic enteritis.
Good hygiene, sanitation, and providing well-timed preventive treatments, especially for pups and kittens, will help to prevent the transmission of intestinal ascarids and hookworms from pets to people.
REF: The University of Georgia Veterinary Newsletter, No. 337, August 1997.
Antidote for Ethylene Glycol Poisoning Approved
For the first time, there is an approved new animal drug for use as an antidote for ethylene glycol poisoning in dogs. Ethylene glycol is a chemical component of antifreeze and coolants and is also used as an industrial solvent for manufacturing detergents, paints, and lacquers. Ethylene glycol is sweet-tasting and dogs will drink it if the opportunity arises. It is highly toxic requiring only a few milliliters (ml) to produce toxicity.
Antizol-Vet™ (fomepizole) injectable was approved by FDA on November 25, 1996. The drug sponsor is Orphan Medical, Inc., Minnetonka, Minnesota. The active ingredient of this product is also known as 4-methylpyrazole. This product may only be used by or on the order of a licensed veterinarian.
REF: The University of Georgia Veterinary Newsletter, No. 337, August 1997.
National Pesticide Telecommunications Network (NPTN)
NPTN is ...
NPTN can ...
NPTN operates ...
Also at NPTN...
The National Antimicrobial Information Network (NAIN), is a toll-free telephone service provided by NPTN. NAIN responds to information requests about antimicrobial products: sanitizers, disinfectants, and sterilants.
NAIN operates -- 7:30 a.m. to 4:30 p.m.,
Pacific time, Monday through Friday, excluding holidays.
Phone: 1-800-447-6349
Fax: 1-541-737-0761
Email: nain@ace.orst.edu
Website: http://ace.orst.edu/info/nain
Art Craigmill
Extension Toxicologist
UC Davis